Cyanoacetamide: A Versatile Compound in Modern Chemistry – Properties, Applications, and Best Practices for Storage
Introduction
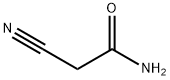
Cyanoacetamide, with the molecular formula C3H4N2O, is a versatile organic compound that has garnered significant attention in the field of chemistry due to its wide range of applications and unique properties. As a key building block in organic synthesis, Cyanoacetamide plays a crucial role in the development of pharmaceuticals, agrochemicals, and dyes, among other products.

Figure 1 Characteristics of Cyanoacetamide
Properties
Cyanoacetamide is a white to off-white crystalline solid that is known for its relatively high melting point, typically ranging between 118°C to 122°C. The compound has a molecular weight of 84.08 g/mol, making it a relatively small and efficient molecule for various synthetic applications.
Chemically, Cyanoacetamide is characterized by the presence of both an amide group (-CONH2) and a cyano group (-CN) attached to a methylene group (-CH2-). This unique combination of functional groups imparts Cyanoacetamide with a high degree of reactivity, making it a valuable intermediate in organic synthesis.
The compound is moderately soluble in water, with a solubility of approximately 15 g/L at room temperature. It is also soluble in many organic solvents, including ethanol, methanol, and acetone. The pH of a Cyanoacetamide solution tends to be slightly acidic, owing to the presence of the amide group, which can donate a proton under the right conditions.
In terms of stability, Cyanoacetamide is generally stable under standard laboratory conditions. However, it is sensitive to strong acids and bases, which can catalyze its decomposition or transformation into other products. Additionally, the compound should be kept away from oxidizing agents, as it may react vigorously with them.
Major Components
Cyanoacetamide, as a single-component compound, primarily consists of carbon, hydrogen, nitrogen, and oxygen atoms. The molecular structure of Cyanoacetamide can be broken down as follows:
Carbon (C): Three carbon atoms form the backbone of the molecule, with one carbon being part of the cyano group, one forming the carbonyl carbon in the amide group, and the third as part of the methylene group.
Hydrogen (H): Four hydrogen atoms are present, two of which are attached to the nitrogen atom in the amide group, while the other two are bonded to the carbon in the methylene group.
Nitrogen (N): Two nitrogen atoms are present, one in the cyano group and the other in the amide group, contributing to the compound’s reactive nature.
Oxygen (O): One oxygen atom is present, which is part of the carbonyl group in the amide functionality.
The interplay of these elements within the Cyanoacetamide molecule results in its distinctive chemical properties, which are leveraged in various industrial and research applications.
Applications
Cyanoacetamide is a compound of considerable interest due to its wide range of applications in different sectors of the chemical industry. Its primary uses include:
Pharmaceuticals: Cyanoacetamide is a key intermediate in the synthesis of various pharmaceuticals, including sedatives, analgesics, and anticonvulsants. Its ability to form carbon-carbon bonds with other organic molecules makes it a valuable precursor in the production of heterocyclic compounds, which are often core structures in many drugs.
Agrochemicals: In the agrochemical sector, Cyanoacetamide is used as a building block for the synthesis of herbicides and insecticides. The compound’s reactivity allows for the creation of complex molecules that can target specific biological pathways in pests and weeds, improving crop yields and reducing the reliance on more toxic chemicals.
Dyes and Pigments: The chemical structure of Cyanoacetamide lends itself to the production of a variety of dyes and pigments. These compounds are used in textiles, inks, and plastics, where Cyanoacetamide-derived dyes offer high stability and vibrant colors.
Organic Synthesis: Beyond its role in specific industries, Cyanoacetamide is widely used in organic synthesis for the preparation of various intermediates and complex molecules. It is particularly valuable in the formation of enamines and heterocycles, which are foundational structures in organic chemistry.
Research and Development: In the realm of academic and industrial research, Cyanoacetamide is frequently employed as a starting material or reagent in the development of new compounds and chemical reactions. Its versatile reactivity and relatively simple structure make it a favorite among chemists exploring novel synthetic pathways.
Storage Requirements
Given its chemical properties, Cyanoacetamide must be stored under specific conditions to ensure its stability and safety. Proper storage is crucial to prevent degradation, contamination, or accidental reactions that could compromise its quality or pose risks to laboratory personnel.
Temperature: Cyanoacetamide should be stored in a cool, dry place, away from sources of heat or ignition. A temperature range of 2°C to 8°C is generally recommended to maintain the compound’s stability over time.
Containers: The compound should be kept in airtight containers made of materials that do not react with it, such as glass or certain types of plastic. This prevents moisture uptake and exposure to air, which could lead to hydrolysis or oxidation.
Light Exposure: Cyanoacetamide is sensitive to light, which can catalyze its degradation. Therefore, it is advisable to store the compound in opaque or amber-colored containers to minimize light exposure.
Handling Precautions: When handling Cyanoacetamide, it is important to use appropriate personal protective equipment (PPE), including gloves, goggles, and lab coats. The compound should be handled in a well-ventilated area, such as a fume hood, to avoid inhalation of dust or fumes.
Segregation: To prevent unwanted reactions, Cyanoacetamide should be stored separately from strong acids, bases, and oxidizing agents. This segregation minimizes the risk of accidental chemical reactions that could be hazardous.
Conclusion
Cyanoacetamide is a compound of significant importance in the field of chemistry, offering a wide range of applications due to its unique properties. From pharmaceuticals and agrochemicals to dyes and organic synthesis, its versatility and reactivity make it an invaluable tool for chemists. However, the proper storage and handling of Cyanoacetamide are essential to maintaining its integrity and ensuring the safety of those who work with it. As research continues to explore new uses and synthetic methods involving Cyanoacetamide, its role in the chemical industry is poised to expand even further.
[1] Fadda A A, Bondock S, Rabie R, et al. Cyanoacetamide derivatives as synthons in heterocyclic synthesis[J]. Turkish Journal of Chemistry, 2008, 32(3): 259-286.
[2] Misic-Vukovic M, Mijin D, Radojkovic-Velicovic M, et al. Condensation of 1, 3-diketones with cyanoacetamide: 4, 6-disubstituted-3-cyano-2-pyridones[J]. JOURNAL-SERBIAN CHEMICAL SOCIETY, 1998, 63: 585-600.
References:
[1] EL-GOHARY N S. Arylidene Derivatives as Synthons in Heterocyclic Synthesis[J]. Open Access Library Journal, 2014, 95 1. DOI:10.4236/OALIB.1100367.[2] M. MISIC‐VUKOVIE. Condensation of 1,3‐Diketones with Cyanoacetamide: 4,6‐Disubstituted‐3‐cyano‐2‐pyridones[J]. ChemInform, 1998, 1 1. DOI:10.1002/CHIN.199848322.
See also
Lastest Price from 2-Cyanoacetamide manufacturers

US $0.00-0.00/KG2025-05-19
- CAS:
- 107-91-5
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10000KGS

US $0.00-0.00/Kg2025-04-21
- CAS:
- 107-91-5
- Min. Order:
- 1Kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons