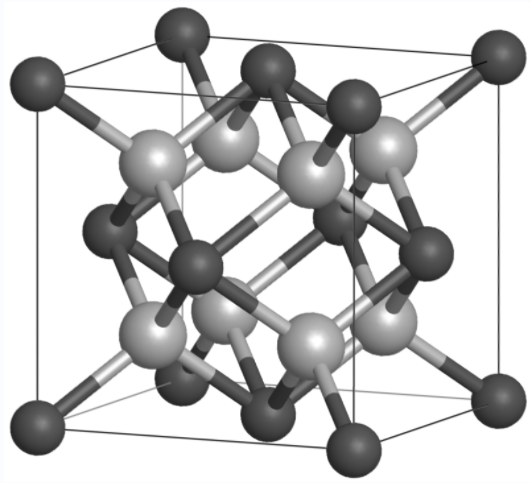
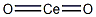Crystal Structure of Cerium dioxide
Cerium dioxide (CeO2) has a fluorite crystal structure, which is a face-centred cubic (FCC) arrangement with a space group of Fm̅3 m. In this structure, the oxygen atoms form a simple cubic sublattice, with cerium ions occupying alternating cubic centres. Each cerium ion is coordinated to eight oxygen atoms, and each oxygen atom is coordinated to four cerium atoms.The single cell of CeO2 consists of a three-dimensional arrangement of these atoms, forming a repeating pattern throughout the crystal. The crystal structure is shown below:
CeO2 is tolerant of a high degree of oxygen deficiency, which is facilitated by the substitution of low-valent elements on the cationic sublattice. This property gives CeO2 a high oxygen ion conductivity and makes it a potential solid electrolyte material for solid oxide fuel cells (SOFCs).The morphology of CeO2 nanoparticles (e.g., nanorods, nanowires, and nanocubes) exposes different crystalline surfaces, which affects their catalytic activity and other properties. There is evidence that the lattice of nanoscale CeO2 expands as the particle size decreases, thus affecting its oxygen storage and release capacity.
[1] R.J. GABORIAUD; B. L; F Paumier. Disorder–order phase transformation in a fluorite-related oxide thin film: In-situ X-ray diffraction and modelling of the residual stress effects[J]. Thin Solid Films, 2016. DOI:10.1016/j.tsf.2015.08.030.
Related articles And Qustion
See also
Lastest Price from Cerium dioxide manufacturers

US $6.00/kg2025-04-21
- CAS:
- 1306-38-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month

US $3.60/kg2025-04-18
- CAS:
- 1306-38-3
- Min. Order:
- 1kg
- Purity:
- ≥99%
- Supply Ability:
- 3000tons/month