Crystal Structure of Boron Phosphide
Boron Phosphide (BP) is a refractory wide bandgap AIIIBV semiconductor with a unique combination of mechanical-thermal and electrical properties, high thermoelectric power, and excellent chemical and high-temperature stability. BP has a polymorphous crystal structure including c-BP (a); w-BP (b); rh-BP (hexagonal axes) - 3D ( c) and 2D (d). A unique interatomic B–P distance of 1.97 Å is identified for all three polymorphs. The specific structures are shown below:
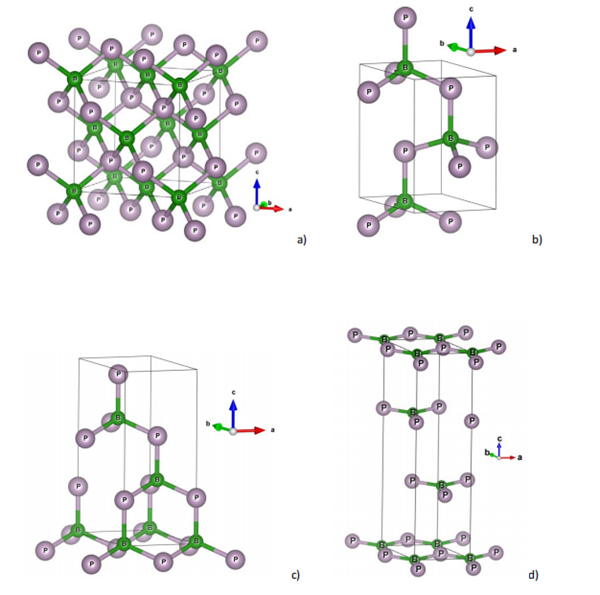
c-BP is a rhodochrosite, sphalerite structure with crystals in the cubic F̅43m space group. b³⁺ is bonded to four equivalent P³- atoms to form a co-angled BP₄ tetrahedron. p³- is bonded to four equivalent B³⁺ atoms to form a co-angled PB₄ tetrahedron.
w-BP is a Wurtzite structure that crystallises in the P6₃mc hexagonal space group. b³⁺ bonds with four equivalent P³- atoms to form the co-angled BP₄ tetrahedron.
It should be noted that the structure of
rhombohedral BP can be considered as three-dimensional (3D) form of a 2D analogue with 3 B-P
layers shown in Fig. 1d. The similar 2D structure has been considered earlier for the study of rhB2N2, a new rhombohedral polymorph of boron nitride.
References:
[1] SOLOZHENKO V, MATAR S. Polymorphism of boron phosphide: Theoretical and experimental assessments[C]. 2022. DOI:10.26434/chemrxiv-2022-kmh23.