Crystal Structure and Properties of Diamond Monocrystalline Powder
Diamond monocrystalline powder is the epitome of purity among crystalline carbon allotropes. It comprises numerous carbon atoms intricately bonded in a tetrahedral fashion. In this arrangement, each carbon atom forms strong bonds with four neighboring carbon atoms, creating a robust three-dimensional lattice structure. Diamond monocrystalline powder owe their unparalleled brilliance to the extraordinary conditions of immense heat and pressure deep within the Earth's crust, where they are formed. These intense forces contribute to the mineral's remarkable luster and shimmer.
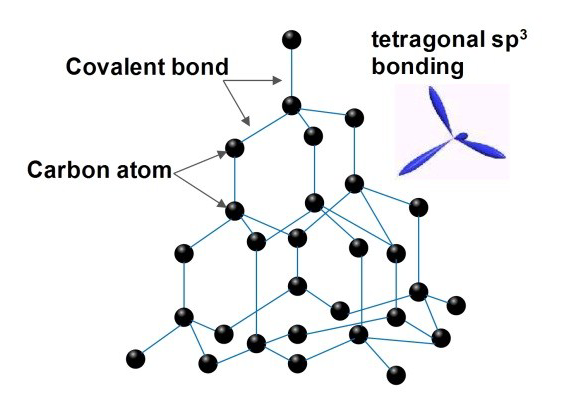
Figure 1. Crystal structure of diamond[1].
Diamond monocrystalline powder is almost entirely made of carbon, with trace amounts of additional elements such as nitrogen and boron, exceptionally chemically stable and does not react with the majority of chemicals, including acids and bases. Because of this, it is an excellent material for use in severe or corrosive situations.
Diamond monocrystalline powder has a low reactivity because it is a poor conductor of electricity and heat and does not react well with many other elements or compounds.
Reference
[1] Mohsin Raza. Synthesis of hydrogenated amorphous carbon (a-C:H) thin films by HiPIMS-based processes. Master Thesis, Linköpings University.