Chemical uses of triethyl orthoformate
Background
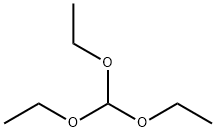
Triethyl orthoformate, also known as triethoxymethane, 1,1',1'' (methine tri(oxo)) triethane, is an organic substance with the chemical formula C7H16O3, a pungent odor liquid[1], low melting and boiling point, miscible with ethanol and ether, slightly soluble in water, mainly used as raw materials for the preparation of antimalarial drugs chloroquine, quinpe and photographic agents, photosensitive materials, and also for the synthesis of formazan and cyanine dyes, etc.

Picture 1 Triethyl orthoformate liquid
Storage Precautions
Triethyl orthoformate should be stored in a cool, ventilated warehouse. Keep away from fire and heat sources[2]. The library temperature should not exceed 37℃. Keep container tightly closed. It should be stored separately from oxidants, acids, etc., and should not be mixed. Use explosion-proof lighting and ventilation facilities. Prohibit the use of mechanical equipment and tools that are prone to sparks. Storage areas should be equipped with emergency release equipment and suitable containment materials
The synthetic method of triethyl orthoformate
Sodium ethoxide method: take sodium ethoxide and chloroform as raw materials, react at 60-65 ℃, generate crude triethyl orthoformate, and then obtain the finished product by fractional distillation.
Ethanol method: replace sodium ethoxide with ethanol and sodium hydroxide, adopt solid-liquid phase transfer catalysis method, and the catalyst is PTC.
Benzoyl chloride method: Using benzoyl chloride, formamide and absolute ethanol as raw materials, under the action of a catalyst, a mixture of triethyl orthoformate, benzoic acid and ammonium chloride is generated, and the finished product triethyl orthoformate is obtained after separation. ethyl ester and benzoic acid.
Put 3 liters of absolute ethanol and 490 g (327 ml, 4.1 moles) of chloroform in a 5 liter round bottom flask equipped with an 80 cm long reflux condenser. The flask should be placed in a position where it is easy to cool with running water. 207 grams (9 gram atoms) of clean sodium metal were cut into small graves so that they could fit into the condenser and added to the solution in about two hours. In order to add metallic sodium at this rate, the flask must be cooled while the sodium is being added. When the sodium metal has been completely used and the mixture has cooled to room temperature, use a drying apparatus to filter off the sodium chloride by suction filtration, wash the sodium oxide on the filter paper with 200 ml of absolute ethanol, and let the washing solution flow into the filtrate obtained above. middle.
The filtrate was placed in a 3 liter flask equipped with an 80 cm fractionation column, and the excess chloroform and most of the ethanol were distilled off on a steam or water bath. The distillate was collected in a 2-liter suction filter bottle equipped with a drying flue to isolate moisture. Distillation takes 5 to 6 hours. The mixture of chloroform and ethanol recovered in the distillation towel weighed about 2000 grams and could be kept for the next experiment. The liquid remaining in the flask was decanted to remove a small amount of sodium fluoride, poured into a Kjeldahl flask with a 30 cm radium column, and distilled under normal pressure. The fraction below 85°C is mainly ethanol, which will be discarded in total; the fraction of 85-140°C is about 100 grams, which contains about a quarter of the total production of ethyl orthoformate. This fraction can be fractionated, but is best combined in the product of the next experiment. The original carbinol is distilled at 140-146 ℃ lbj, weighing 120-140 grams (27-31%).
After the first experiment, 400 grams of chloroform was added to the recovered chloroform-ethanol mixture with enough anhydrous ethyl alcohol (800-1000 mL) to bring the total to 3 liters. Then add sodium metal with the previous shot. After the excess chloroform and ethanol had been distilled from the distillation column, the middle portion of the last experiment was added, followed by fractional distillation. The yield of this experiment was about 200 grams 45%.
Chemical uses of triethyl orthoformate
Triethyl orthoformate is a commonly used alkylating and formylating reagent in chemical laboratories. They are easy to store but highly reactive. They can be used as alkylating reagents, which can easily transfer the alkyl group to various alcohol hydroxyl groups; when used as formylation reagents, they can be used under acidic or basic conditions. Alkylation reaction with alcohol When triethyl orthoformate is used as an alkylating agent, various alcohols can undergo corresponding alkylation reaction. The polyols can be alkylated to form the corresponding cyclic orthoformates. Protected orthoformates of aldehydes or ketones can be used to protect aldehydes and ketones to generate formate esters and the corresponding acetals or ketals.
Formylation Reaction Under the catalysis of Lewis acid, orthoformate can be used as formylation reagent. Compared with other formylation reagents, it has the characteristics of simple operation and high yield. Under acid catalysis, orthoformate easily generates dialkoxycarbon ions in situ, which can be directly used in formylation. Such formylation reactions can be at α-C ortho to the carbonyl group. Reaction with Enols (or the like) Enolates of silyl ethers or ketone enolates can react with carbonium ions generated in situ from orthoformates to form β-acetal ketones.
For organic synthesis and as pharmaceutical intermediates and photosensitive materials. Triethyl orthoformate is an intermediate of the acaricide amitraz and the herbicide pyrazosulfuron, and can also be used to make antimalarial drugs such as chloroquine and quinoper. Triethyl orthoformate is an important pharmaceutical raw material, and is a synthetic raw material for antimalarial drugs such as chloroquine and quinoper. It is also used to make polymers, photographic medicines, photosensitive materials, anti-halation dyes, cyanine dyes and synthetic pesticides, etc.
Reference
1 Zhang Mingsen, etc. The Complete Book of Fine Organic Chemical Intermediates: Chemical Industry Press, 2008
2 Dictionary of Organic Synthesis Beijing Institute of Technology Press Fan Nengting 1995
See also
Lastest Price from Triethyl orthoformate manufacturers

US $0.00-0.00/kg2025-04-21
- CAS:
- 122-51-0
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons
US $0.00/Kg/Drum2025-04-21
- CAS:
- 122-51-0
- Min. Order:
- 180KG
- Purity:
- 99.4
- Supply Ability:
- 1000kg