Chemical properties of Polyvinylpyrrolidone
Description
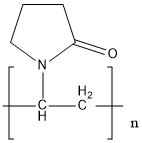
Polyvinylpyrrolidone, or PVP, is one of the most essential fine chemicals derived from acetylene. Molecular formula is (C6H9NO)n. PVP is tasteless, odorless, and low toxicity. It has excellent physiological inertia and biocompatibility and has no irritation to the skin and eyes. Commercial PVP is a solid white or milky white powder.

K-value and molecular weight
Due to the difference in n number (the degree of polymerization), PVP has different molecular weights and slightly different properties. PVP with different molecular weight ranges is generally expressed by K-values (e.g., K-12, K-15, K-17, K-30, K-60, and K-90).
Solubility
One of the outstanding properties of PVP is its universal solubility in hydrophobic and extremely hydrophilic solvents. PVP is generally easier to dissolve in more polar solvents but difficult to dissolve in weakly polar or nonpolar solvents. However, when a co-solvent is present, the solubility of PVP in weakly polar or nonpolar solvents can be increased. Because the molecular structure of PVP has hydrophilic imido groups and lipophilic alkyl groups, it can be dissolved in water and many organic solvents. PVP is completely miscible in water and some ordinary organic solutions such as ethanol and isopropanol. However, the solubility of PVP in an etone is as small as only 1–2%.
Surface activity
The molecular structure of PVP contains a strong polar acylamino group with a dipole distance of 40 C · m, which has the ability of hydrophilic and affinity polar groups, and its nonpolar alkyl group makes it lipophilic. The end of the lactam-based oxygen atom with the larger dipole moment is bare, while the end of the nitrogen atom is surrounded by methyl and methylene groups. The molecular structure of PVP makes it surface-active. As a polymer compound, it can widely adjust the rheological properties of dispersions or solutions. Its hydrogen bonding ability with many inorganic compounds and organic compounds provides excellent coagulant on or solubilization effect; the former makes it clarify and stabilize in alcohol and beverages containing polyphenols, while the latter is widely applied in coprecipitation to prepare drug solid dispersions for improved solubility of poorly soluble drugs. Therefore, PVP has become one of the main varieties of polymer surfactants.
Complexation
The PVP molecule has a strong polar amide group and can bond with hydrogen, which allows it to have a strong complexing ability with many substances, especially compounds containing hydroxyl, carbonyl, carboxyl, amino, and other active hydrogen atoms. PVP forms solid complexes with iodine, β-carotene, reserpine, tolbutamide, phenytoin, amorphous e, griseofulvin, and various sulfonamide drugs. After complexation, the thermodynamic activity of these small molecular substances is reduced, and the stability and solubility of water are improved. For example, iodine is easily sublimated, insoluble in water, and easily soluble in chloroform. However, the complex of PVP and iodine (PVP-I) is easily soluble in water, nonvolatile, and even unable to be extracted by chloroform. The complexation of PVP has been widely used in medicine, and many insoluble drugs become soluble after complexing with PVP.
The complexes formed by PVP and polyphenols are usually insoluble in water. The stability of these complexes is related to the pH of the solution. Oxygen and nitrogen atoms in PVP molecules are typical coordination atoms, making them capable of forming complexes with many metals. Among them, the most studied are transition metals and precious metals. For example, PVP and Fe, Mn, Co, Ni, and other transition metals form complexes.
Chemical stability
Under normal circumstances, PVP solids are very stable, with no change after heating at 100 °C for 16 h. If heated to 150 °C or mixed with an initiator such as ammonium persulfate and heated at 90 °C for 30 min, a c oss-linking reaction occurs, and it becomes an insoluble cross-linked solid. In the presence of azo compounds, irradiation with ultraviolet light and gamma rays will produce a stable gel. Long-term grinding will lead to the degradation of PVP, and the average molecular weight will decrease. The aqueous solution of PVP is usually very stable, and there is no obvious change when heated to 100 °C, yet the color of the solution becomes light yellow after heating for a long time. Adding some salts containing polyvalent anions to the PVP aqueous solution, such as sodium tripolyphosphate and sodium metasilicate, will cause precipitation.
Physiological inertness and safety
Many toxicological and biochemical studies show the excellent physiological inertness and biocompatibility of PVP. The acute, subchronic, and chronic toxicity of PVP orally or intraperitoneally is extremely low, with the only effect observed being diarrhea at high doses due to its osmotic action. LD50 values of PVP range from 12 g kg–1 intraperitoneally in the mouse to 100 g kg–1 orally in the rat, suggesting that it belongs to the actual nontoxic level according to the oral toxicity classification standard. PVP has no irritating or allergic effects on humans skin, mucous membranes, and eyes. Neither carcinogenic effect nor reproductive toxicity has been found following oral administration or intraperitoneal, subcutaneous, and intravenous injections. Pharmacological studies have demonstrated that PVP has no effect on the central nervous system, respiratory system, and blood circulation system.
PVP is not metabolized in the body. It is not absorbed by the gastrointestinal tract. No gastrointestinal toxicity has been reported. PVP with a lower molecular weight can be completely excreted in urine within a few days. PVP with a higher molecular weight will slowly accumulate in the body, mainly in reticuloendothelial tissue cells, especially temporarily in the spleen, liver, lymph nodes, bone marrow, and kidneys. The degree of storage and disappearance is related to molecular weight, dosage, and duration of intake. However, this accumulation did not cause damage or harmful effects on tissue morphology or function. Non-gastrointestinal administration studies have shown that PVP has no carcinogenic effect, and some experiments even observed that PVP has a specific tumor-suppressing effect.
In parenteral administration, PVP with low molecular weight is easily excreted from the renal system, while PVP with high molecular weight is excreted slowly. Therefore, it must be noted that when PVP is used as an injection to directly enter human blood, its molecular weight is an essential factor that must be considered. PVP does not maintain its original compact configuration like protein in an aqueous solution but exists in water like a polymer with flexible chains in a scattered spiral configuration, and its size depends on molecular weight. In saline, the sizes of PVP molecules with different molecular weights are between 1 and 100 nm. The inner diameter of the human kidney capillary is about 7 nm; that is, the molecular size of PVP entering the human blood circulation system should be less than 7 nm to be excreted smoothly through the kidney. The molecular weight corresponding to the 7 nm size is about 30,000; that is, the molecular weight of PVP as an injection component should be less than 30,000. Polymer products are characterized by average molecular weight, and there is also the problem of molecular weight distribution. Therefore, to ensure that PVP products used for injections do not contain components with molecular weights greater than 30,000, the average molecular weight is required to be much less than 30,000.
Related articles And Qustion
See also
Lastest Price from Polyvinylpyrrolidone manufacturers

US $10.00-8.00/kg2025-07-18
- CAS:
- 9003-39-8
- Min. Order:
- 500kg
- Purity:
- 95-99%
- Supply Ability:
- 20 tons

US $22.00-5.00/kg2025-06-25
- CAS:
- 9003-39-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000kg