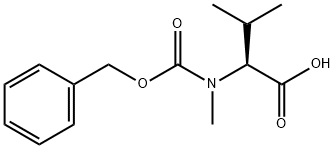
Cbz-N-methyl-L-valine - Reaction / Application on synthetic works
(S)-N-(Benzyloxycarbonyl)-N-methylvaline has been used as a reactant in the synthesis of a biologically active peptide. It is an important organic intermediate to synthetize substituted valine products.
The following example is about its application on the synthesis of the drug linker[1]

The Cbz-N-methyl-L-valine (2.65 g, 10.0 mmol), HATU (4.56 g, 12.0 mmol) and DIEA (3.87 g, 30.0 mmol) is added to dichloromethane (50 ml), stirring at the room temperature under the protection of nitrogen reaction 30 minutes, and then adding the amine (2.09 g, 10.0 mmol), stirring at the room temperature under the protection of nitrogen reaction 4 hours, LCMS display in a reaction Cbz-N-methyl-L-valine less than 3% as the end of the reaction. The reaction solution for sequentially aqueous solution of citric acid (20 ml), saturated salt water (20 ml) washing, anhydrous sodium sulfate drying, turns on lathe does, crude product by 20 ml (petroleum ether: ethyl acetate=10:1) mixed solvent pulping, filtering, the resulting solid petroleum ether (10 ml) washing, a ground line, get the product (white solid, 3.92 g, yield 93%).
The following example is about its application on the synthesis of a cleavable linker [2]
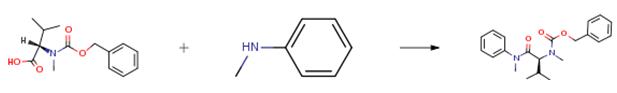
To a solution of z-Valine (1.01 g, 3.81 mmol) and N-methylaniline (412 1iL, 3.81 mmol) in DCM (15 mL) was added DCC (1.18 g, 5.71 mmol) and DMAP (92 mg, 0.76 mmol) at room temperature under N2 atmosphere, followed by stirring at room temperature for 3 hours. The mixture was filtered through Celite and concentrated under reduced pressure. The residue was purified by column chromatography to obtain the product (1.05 g, 78 percent).
The following example is about its application on the synthesis of substituted phenethylamine derivatives [3]
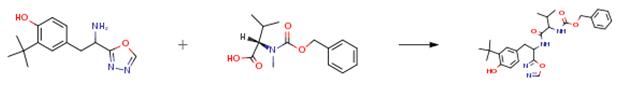
To a solution of Z-N-Me-Val-OH (914 mg, 3.45 mmol), 2-(3-t-butyl-4-hydroxyphenyl)-1-(1,3,4-oxadiazol-2-yl)ethylamine (0.75 g, 2.87 mmol) and CMPI (881 mg, 3.45 mmol) in THF (30 ml), TEA (0.96 ml) was added under cooling with ice and stirred at room temperature for 2 hours. The reaction mixture was mixed with water and extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate and evaporated to remove the solvent under reduced pressure; the thus obtained residue was subjected to silica gel column chromatography (developing solvent: ethyl acetate:n-hexane = 1:1), giving 2-benzyloxycarbonylamino-3-methylbutyric acid 2-(3-t-butyl-4-hydroxyphenyl)-1-(1,3,4-oxadiazol-2-yl)ethylamide (1.28 g, 88 percent).
The following example is about its application on the synthesis of phenethylamine derivatives [3]
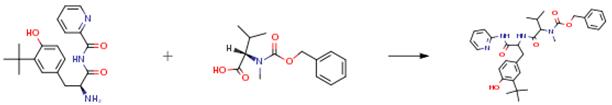
To a solution of 3-tert-butyl-4-hydroxyphenylalanyl (2-pyridyl)amide (1.48 g, 4.73 mmol), Z-N-Me-Val-OH (1.63 g, 6.15 mmol) and CMPI (1.57 g, 6.15 mmol) in THF 30 ml, TEA (1.5 ml, 10.88 mmol) was added under cooling with ice and stirred for 3 hours under cooling with ice. The mixture was mixed with water and extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate and evaporated to remove the solvent under reduced pressure; the thus obtained residue was subjected to silica gel column chromatography (developing solvent: ethyl acetate:n-hexane = 1:2), giving the product (1.74 g, 65 percent).
References
1.Lianning (Suzhou) Bio-pharmaceutical Co., Ltd. Xu Z, Li H, Guo M, Li H. For antibody drug conjugates of the drug - linker MC - MMAF preparation method and intermediate (by machine translation). CN109928908[P], 2019, A, Paragraph 0082-0085.
2.Intocell I, Park T, Woo SH, Kim S, Park S, Cho J, Jung D, Park O, Seo B. Compounds comprising cleavable linker and uses thereof. WO2019/8441[P], 2019, A1, Page column 174; 175.
3.Chugai Seiyaku Kabushiki Kaisha. Substituted phenethylamine derivatives. EP1149843[P], 2001, A1
You may like
Lastest Price from Cbz-N-methyl-L-valine manufacturers
US $1.00/g2020-01-06
- CAS:
- 42417-65-2
- Min. Order:
- 10g
- Purity:
- 98%
- Supply Ability:
- 100kg

![125248-71-7 1,4-bis-[4-(6-acryloyloxyhexyloxy)benzoyloxy]-2-methylbenzene; preparation; synthesis](httpss://www.chemicalbook.com/NewsImg/2019-11-27/20191127943334412.jpg)