Cannabidiol: pharmacology, clinical applications and toxicity
General Description
Cannabidiol is a non-psychoactive compound derived from the cannabis plant. It has shown promise in various medical conditions. CBD has been proven effective in reducing seizures in epilepsy patients and has gained regulatory approval for certain forms of epilepsy treatment. Additionally, CBD has potential in managing chronic pain, anxiety disorders, and symptoms of neurological disorders and cancer. However, CBD does have potential adverse effects at high doses, including developmental toxicity and hepatocellular injuries. Common side effects include drug interactions, hepatic abnormalities, diarrhea, fatigue, vomiting, and drowsiness. Further research is needed to fully understand the long-term consequences and establish proper guidelines for cannabidiol use.

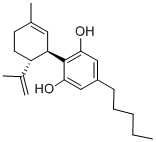
Figure 1. Cannabidiol
Pharmacology
Cannabidiol exhibits complex pharmacology as a therapeutic drug. Its pharmacokinetic profile includes low bioavailability and significant protein binding capability (99% protein binding). Cannabidiol interacts with various metabolic pathways in the liver, including CYPs (cytochrome P450 enzymes) that can be influenced by genetic factors and drug interactions. However, CBD is rapidly cleared from the body due to its interaction with multiple enzymes, making it less susceptible to modulation by drugs affecting metabolizing enzymes. The bioavailability of CBD in oral oil formulations is limited (<6%) due to extensive first-pass metabolism in the liver. Cannabidiol may interact with antiepileptic drugs (AEDs), both as potent enzyme inducers (e.g., carbamazepine and phenytoin) and inhibitors (e.g., stiripentol, felbamate, and valproate). However, the clinical significance of these interactions may not be substantial. One notable interaction is between CBD and clobazam, where CBD inhibits the enzyme responsible for metabolizing clobazam, leading to increased levels of its metabolite and potential toxicity. Concurrent use of clobazam may also enhance the effect of cannabidiol. 1
Clinical application
Cannabidiol has gained significant attention in the field of clinical application due to its potential therapeutic properties. CBD is a non-psychoactive compound derived from the cannabis plant and has shown promise in various medical conditions. One of the primary clinical applications of CBD is in the management of epilepsy. Numerous studies have demonstrated its effectiveness in reducing the frequency and severity of seizures in patients with different types of epilepsy, including Dravet syndrome and Lennox-Gastaut syndrome. In fact, CBD-based medications, such as Epidiolex, have been approved by regulatory authorities for the treatment of certain forms of epilepsy. Beyond epilepsy, cannabidiol has also shown potential in managing chronic pain. It interacts with the endocannabinoid system in the body, which plays a crucial role in regulating pain sensation. CBD's anti-inflammatory and analgesic properties make it an attractive option for individuals suffering from conditions such as arthritis, multiple sclerosis, and neuropathic pain. Moreover, cannabidiol has exhibited promising results in addressing anxiety disorders. Research suggests that CBD can modulate serotonin receptors in the brain, which are involved in regulating mood and anxiety. Studies have reported reduced anxiety levels in individuals with social anxiety disorder, post-traumatic stress disorder (PTSD), and generalized anxiety disorder (GAD) after cannabidiol administration. 2
Toxicity
Cannabidiol is not entirely without risks. Animal studies have revealed several adverse effects of cannabidiol, including developmental toxicity, embryo-fetal mortality, inhibition and neurotoxicity in the central nervous system, hepatocellular injuries, reduction in spermatogenesis, alterations in organ weight, changes in the male reproductive system, and hypotension. However, it is important to note that these effects were observed at doses higher than those typically recommended for human pharmacotherapies. In human studies focused on epilepsy and psychiatric disorders, cannabidiol has been associated with certain side effects. These include drug-drug interactions induced by cannabidiol, hepatic abnormalities, diarrhea, fatigue, vomiting, and somnolence or drowsiness. It is crucial to consider these potential adverse reactions when using cannabidiol as a therapeutic agent. While cannabidiol has shown promising effects in various medical conditions, it is vital to understand and monitor its potential toxicological effects. Further research is necessary to better comprehend the long-term and cumulative consequences of cannabidiol use and to establish appropriate guidelines for its safe and effective utilization. 3
Reference
1. Britch SC, Babalonis S, Walsh SL. Cannabidiol: pharmacology and therapeutic targets. Psychopharmacology (Berl). 2021 Jan;238(1):9-28.
2. Arzimanoglou A, Brandl U, Cross JH, Gil-Nagel A, Lagae L, Landmark CJ, Specchio N, Nabbout R, Thiele EA, Gubbay O, The Cannabinoids International Experts Panel; Collaborators. Epilepsy and cannabidiol: a guide to treatment. Epileptic Disord. 2020 Feb 1;22(1):1-14.
3. Huestis MA, Solimini R, Pichini S, Pacifici R, Carlier J, Busardò FP. Cannabidiol Adverse Effects and Toxicity. Curr Neuropharmacol. 2019;17(10):974-989.