Calcium oxalate: Chemical properties, Uses, Calcium oxalate stones
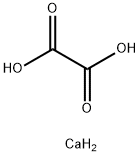
Calcium oxalate is a calcium salt of oxalate with the chemical formula CaC2O4 and molecular weight of 128.10. The monohydrate is a colorless crystal with a relative density of 2.2 and loses moisture when heated to 200 ° C. Hard to dissolve in water and acetic acid, soluble in nitric acid and hydrochloric acid. It breaks down into calcium carbonate and calcium oxide when burned.
Uses
Calcium oxalate is used as an analytical reagent, and a carrier in the separation of rare earth metals. It is also used in the manufacture of ceramic glazing and the preparation of oxalic acid and organic oxalate. Industrially, it is used in the manufacture of lubricating esters and waterproof agent.
Calcium oxalate stones
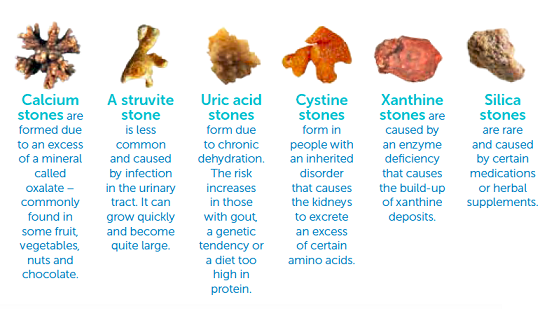
Calcium oxalate stones are also called lithangiuria. Studies have shown that 70% to 80% of the stones in patients with lithangiuria, are mainly composed of calcium oxalate.
Many plants accumulate oxalic acid which easily enters the body through the respiratory tract, digestive tract and skin mucosa. Because of its strong acid nature, administration of 2 grams to 5 grams for adults may be fatal. Scientific research has shown that when gestational ewes are fed 6 g to 12 g of oxalic acid per day, the oxalic acid can pass through the placental barrier and lead to oxalate crystal deposition in the kidney of lamb.
It has been found by the medical circle that some women limit their access to meat and staple foods for a long time, and mainly have fruits and vegetables during their analysis of the etiology of adult urinary calculi. The excessive oxalic acid in fruits and vegetables prompts them to suffer from urinary tract stones. The oxalate stones are difficult to dissolve by themselves once formed.
To prevent the formation of calcium oxalate stones, attention must be paid to adjusting the diet structure, not only reducing the oxalic acid in the food, but also avoiding food combinations with high oxalic acid and high calcium content, such as spinach, soy products with high calcium content and milk that are easy to form calcium oxalate when eating together. When the ratio of calcium to oxalic acid is 1:2, it is very easy to form calcium oxalate stone. If they must be eaten this way, then soak the high oxalic acid vegetables in boiling water for 1 minute, and eat after the oxalic acid is dissolved in water. People, who have to take calcium or calcium supplement, should use it with more care. Apart from the above treatment of high oxalic acid food, it is better to have a 2-hour interval between meal and taking medicine.
In addition, xylitol eventually metabolizes into oxalic acid and is excreted by the kidneys. If a large amount of xylitol is taken in a short time or for a long time, serum oxalic acid levels may suddenly or slowly rise, and crystal deposition is likely to occur after physiological saturation, which may lead to kidney damage and even acute kidney failure.
Referrence
SCHEMEL, J.H. (1977) Manual on Zirconium and Hafnium. STP 639 American Society for Testing and Materials
(ASTM), West Conshohocken, PA,.
THOMAS, D.E.; HAYES, E.T. (1960) The Metallurgy of Hafnium. Naval Reactors, Division of Reactor Development,
US Atomic Energy Commission.
WEBSTER, R.T. (1995) Zirconium and Hafnium. In: ASM Metals Handbook, 9th ed. Vol. 2: Properties and
Selection of Nonferrous Alloys and Special Purpose Materials. ASM, Materials Park OH, pp. 661–721.
See also
Lastest Price from Calcium oxalate manufacturers

US $1.00/KG2025-04-21
- CAS:
- 563-72-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt
US $0.00-0.00/KG2025-03-03
- CAS:
- 563-72-4
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10000KGS