Cadmium:Discovery,Mining,Uses,Health hazards
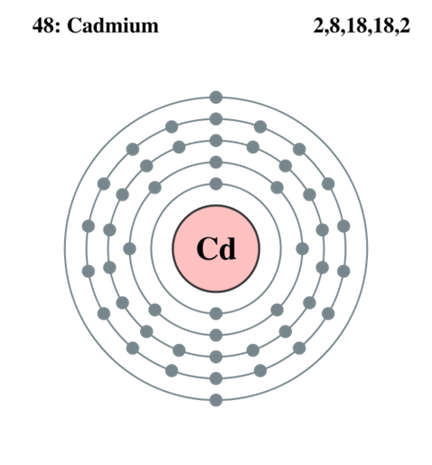
Cadmium is a soft, silvery-white metal. It has 48 electrons arranged in an electronic configuration of [Kr]4d105s2. The two outermost electrons participate in reactions and cadmium usually has an oxidation state of 12 but also exists in the 11 state. Cadmium makes up about 0.1 ppm of Earth’s crust. It is far rarer than zinc, with which it is commonly found and makes up about 65 ppm.
Discovery
Cadmium was discovered at the same time in 1817 by German chemists Friedrich Stromeyer (August 2, 1776 to August 18, 1835) and Karl Samuel Leberecht Hermann (January 20, 1765 to September 1, 1846), as an impurity in zinc carbonate (calamine, now known as smithsonite, ZnCO3), and, for a century, Germany was the only significant producer of the metal (Hermann, 1818). The metal was named after the Latin word for calamine, as it was originally discovered in this zinc ore.
Mining
The only cadmium mineral of significance, greenockite (CdS)(Fig.1), is almost always found together with sphalerite (ZnS). This association is due to the the geochemical similarity between zinc and cadmium, with no probable geological process to separate them. Consequently, cadmium is produced primarily as a by-product of mining, smelting, and refining sulfidic ores of zinc, and, to a lesser degree, lead and copper. Rocks mined for phosphate fertilizers comprise fluctuating amounts of cadmium, resulting in a cadmium concentration of as high as 300 ppm in the fertilizers and a high cadmium concentration in agricultural soils. Coal can have substantial concentrations of cadmium, which ends up generally in flue dust. Cadmium is a commonly occurring impurity in many zinc ores, and it is most often separated during the production of zinc.

Fig.1 greenockite
Major minerals
Only 27 minerals are known to contain cadmium. The native metal cadmium (Cd) is the only mineral in the element class. Eleven different minerals are found in the sulfide class, for example, greenockite (CdS) and quadratite (Ag(Cd, Pb)AsS3). One oxide, monteponite (CdO), is known to contain Cd. A single carbonate, otavite (CdCO3)(Fig.2), is known as Cd.

Fig.2 otavite
Uses
An additional type of battery is the Ag-Cd battery. Cd electroplating, using around 6% of worldwide Cd production, is employed in the aircraft industry to decrease corrosion of steel components. Cd is employed in the control rods of nuclear reactors, serving as a very efficient “neutron poison” to regulate the neutron flux in nuclear fission. As Cd rods are inserted in the core of a nuclear reactor, the Cd absorbs neutrons, stopping them from producing additional fission events, and hence regulating the amount of reactivity.
Health hazards
Cadmium has no known function in higher organisms and is considered toxic. Cadmium is considered an environmental pollutant hazardous to living organisms. A cadmium-dependent carbonic anhydrase has been found in some marine diatoms, which live in environments with low zinc concentrations.Cadmium is under research for its potential toxicity to increase the risk of cancer, cardiovascular disease, and osteoporosis.