Cabozantinib Malate and Cabozantinib
Introduction
Cabozantinib was first approved in 2012 and is a non-specific tyrosine kinase inhibitor. It was initially approved in the US under the brand name Cometriq, which is indicated for treating metastatic medullary thyroid cancer. In 2016, a capsule formulation (Cabometyx) was approved for the treatment of advanced renal cell carcinoma, and this same formulation gained additional approval in both the US and Canada in 2019 for treating hepatocellular carcinoma in previously treated patients.
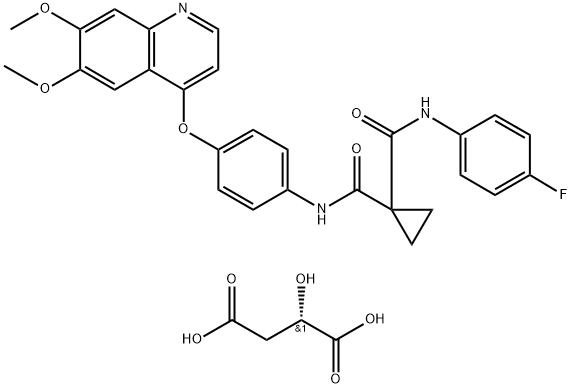
After metabolism, the prodrug cabozantinib malate (CBZM) breaks down into the active metabolite cabozantinib. It is a recently identified small molecule tyrosine kinase inhibitor (smTKI) that targets a particular tissue. The smTKIs are utilized to treat cancer cells because they are overexpressed in malignant cells. It has been suggested for the treatment of advanced renal carcinoma (ARC), castration-resistant prostate cancer (CRPC), medullary thyroid cancer (MTC), and hepatocellular carcinoma (HCC). It is offered for treating MTC, CRPC, HCC, and ARC as commercial oral capsules (Cometriq®) or tablets (Cabometyx®). Commercial CBZM capsules and tablets have been found to have very low bioavailability in both animals and humans, likely due to their poor solubility in an aqueous medium such as water (H2O). The design of CBZM formulations is difficult due to its poor water solubility. Poor bioavailability after oral administration and poor dissolution rate in dosage forms are the main difficulties of CBZM.
Uses in cancer
Cabozantinib-s-malate (Cabozantinib Malate) is approved to treat:
Differentiated thyroid cancer that has spread. It is used in adults and children aged 12 years and older who cannot receive radioactive iodine and whose cancer got worse after VEGF receptor-targeted therapy. This use is approved for the Cabometyx brand of cabozantinib-s-malate.
Hepatocellular carcinoma (a type of liver cancer). It is used in patients who have already been treated with sorafenib. This use is approved for the Cabometyx brand of cabozantinib-s-malate.
Medullary thyroid cancer has gotten worse and has spread to other parts of the body. This use is approved for the Cometriq brand of cabozantinib-s-malate.
Renal cell carcinoma (a type of kidney cancer) is advanced. It is either used with nivolumab as the first therapy or alone. This use is approved for the Cabometyx brand of cabozantinib-s-malate.
Cabozantinib-s-malate is also being studied in the treatment of other types of cancer.
CABOMETYX
CABOMETYX is the (S)-malate salt of cabozantinib, a kinase inhibitor. Cabozantinib (S)-malate (Cabozantinib Malate) is described chemically as N-(4-(6,7-dimethoxyquinolin-4-yloxy)phenyl)-N’-(4 fluorophenyl)cyclopropane-1,1-dicarboxamide, (2S)-hydroxybutanedioate. The molecular formula is C28H24FN3O5·C4H6O5, and the molecular weight is 635.6 Daltons as malate salt. Cabozantinib (S)-malate salt is a white to off-white solid practically insoluble in aqueous media. CABOMETYX (cabozantinib) tablets for oral use are supplied as film-coated tablets containing 20 mg, 40 mg, or 60 mg of cabozantinib, which is equivalent to 25 mg, 51 mg, or 76 mg of cabozantinib (S)-malate, respectively. CABOMETYX also contains the inactive ingredients: microcrystalline cellulose, lactose anhydrous, hydroxypropyl cellulose, croscarmellose sodium, colloidal silicon dioxide, and magnesium stearate.
References:
[1] FAIYAZ SHAKEEL. Solubility and Thermodynamics Data of Cabozantinib Malate in Various Aqueous Solutions of Dimethyl Sulfoxide at Different Temperatures.[J]. Molecules, 2023, 28 23. DOI:10.3390/molecules28237805.See also
Lastest Price from Cabozantinib Malate manufacturers

US $0.00/kg2025-04-20
- CAS:
- 1140909-48-3
- Min. Order:
- 1kg
- Purity:
- 99% HPLC
- Supply Ability:
- 1000kg

US $40.00/kg2025-03-07
- CAS:
- 1140909-48-3
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10 tons