Boron: Production, applications and biological role
General description
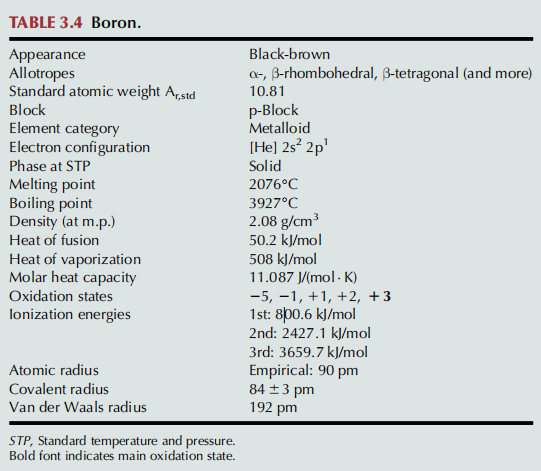
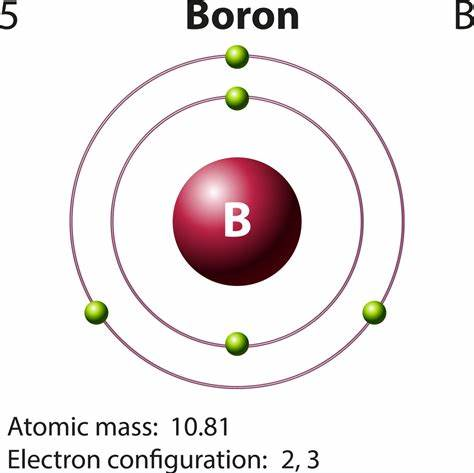
Boron is a chemical element with the symbol B and atomic number 5. Produced entirely by cosmic ray spallation and supernovae and not by stellar nucleosynthesis, it is a low-abundance element in the Solar System and in the Earth's crust. It constitutes about 0.001 percent by weight of Earth's crust. Boron is concentrated on Earth by the water-solubility of its more common naturally occurring compounds, the borate minerals. These are mined industrially as evaporites, such as borax and kernite. The largest known boron deposits are in Turkey, the largest producer of boron minerals. Elemental boron is a metalloid that is found in small amounts in meteoroids but chemically uncombined boron is not otherwise found naturally on Earth. Industrially, very pure boron is produced with difficulty because of refractory contamination by carbon or other elements. Several allotropes of boron exist: amorphous boron is a brown powder; crystalline boron is silvery to black, extremely hard (about 9.5 on the Mohs scale), and a poor electrical conductor at room temperature. The primary use of elemental boron is as boron filaments with applications similar to carbon fibers in some high-strength materials. Its appearance is as follows:

Figure 1 Appearance of Boron
Production
Economically important sources of boron are the minerals colemanite, rasorite (kernite), ulexite and tincal. Together these constitute 90% of mined boron-containing ore. The largest global borax deposits known, many still untapped, are in Central and Western Turkey, including the provinces of Eskişehir, Kütahya and Balıkesir.[1] Global proven boron mineral mining reserves exceed one billion metric tonnes, against a yearly production of about four million tonnes.[67] Turkey and the United States are the largest producers of boron products. Turkey produces about half of the global yearly demand, through Eti Mine Works (Turkish: Eti Maden İşletmeleri) a Turkish state-owned mining and chemicals company focusing on boron products. It holds a government monopoly on the mining of borate minerals in Turkey, which possesses 72% of the world's known deposits.[2] In 2012, it held a 47% share of production of global borate minerals, ahead of its main competitor, Rio Tinto Group.
Applications
Nearly all boron ore extracted from the Earth is destined for refinement into boric acid and sodium tetraborate pentahydrate. In the United States, 70% of the boron is used for the production of glass and ceramics.[3] The major global industrial-scale use of boron compounds (about 46% of end-use) is in production of glass fiber for boron-containing insulating and structural fiberglasses, especially in Asia. Boron is added to the glass as borax pentahydrate or boron oxide, to influence the strength or fluxing qualities of the glass fibers. Another 10% of global boron production is for borosilicate glass as used in high strength glassware. About 15% of global boron is used in boron ceramics, including super-hard materials discussed below. Agriculture consumes 11% of global boron production, and bleaches and detergents about 6%. Boron fibers (boron filaments) are high-strength, lightweight materials that are used chiefly for advanced aerospace structures as a component of composite materials, as well as limited production consumer and sporting goods such as golf clubs and fishing rods. The fibers can be produced by chemical vapor deposition of boron on a tungsten filament.
Biological role
Boron is an essential plant nutrient, required primarily for maintaining the integrity of cell walls. However, high soil concentrations of greater than 1.0 ppm lead to marginal and tip necrosis in leaves as well as poor overall growth performance. Levels as low as 0.8 ppm produce these same symptoms in plants that are particularly sensitive to boron in the soil. Nearly all plants, even those somewhat tolerant of soil boron, will show at least some symptoms of boron toxicity when soil boron content is greater than 1.8 ppm. When this content exceeds 2.0 ppm, few plants will perform well and some may not survive.[4] It is thought that boron plays several essential roles in animals, including humans, but the exact physiological role is poorly understood.[5] A small human trial published in 1987 reported on postmenopausal women first made boron deficient and then repleted with 3 mg/day. Boron supplementation markedly reduced urinary calcium excretion and elevated the serum concentrations of 17 beta-estradiol and testosterone.
References
[1]Kistler, R. B. (1994). "Boron and Borates" (PDF). Industrial Minerals and Rocks (6th ed.): 171–186.
[2]Kistler, R. B. (1994). "Boron and Borates" (PDF). Industrial Minerals and Rocks (6th ed.): 171–186.
[3]Hammond, C. R. (2004). The Elements, in Handbook of Chemistry and Physics (81st ed.). CRC press. ISBN 978-0-8493-0485-9.
[4]Blevins, Dale G.; Lukaszewski, K. M. (1998). "Functions of Boron in Plant Nutrition". Annual Review of Plant Physiology and Plant Molecular Biology. 49: 481–500. doi:10.1146/annurev.arplant.49.1.481. PMID 15012243.
[5]Nielsen, Forrest H. (1998). "Ultratrace elements in nutrition: Current knowledge and speculation". The Journal of Trace Elements in Experimental Medicine. 11 (2–3): 251–274. doi:10.1002/(SICI)1520-670X(1998)11:2/3<251::AID-JTRA15>3.0.CO;2-Q.
You may like
Related articles And Qustion
See also
Lastest Price from Boron manufacturers

US $10.00-7.00/kg2025-02-13
- CAS:
- 7440-42-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 tons

US $0.00-0.00/g2024-09-25
- CAS:
- 7440-42-8
- Min. Order:
- 50g
- Purity:
- 99%
- Supply Ability:
- 60kg