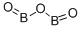Boron Oxide: Unique Characteristics, Applications in Lithium-Ion Batteries and Environmental Risk
General Description
Boron oxide, a compound with unique chemical and physical properties, is used in various industries due to its stability, dehydrating capabilities, and electrical insulation properties[1].

Uses
Recently, lithium nickel cobalt manganese oxide (NMC) cathode materials have effectively enhanced their stability by using boron compounds, such as cobalt boride (CoxB) and B2O3, for surface modifications. Yoon et al. revealed that CoxB metallic glass in Ni-rich NMC can effectively enhance the stability of cathode materials via reactive wetting. Li et al. utilized B2O3 as a surface-modification material to enhance the performance of the NMC111 cathode. The use of B2O3 also resulted in graphene combined with a MoS2 hierarchical structure, which improved the photo/electro properties of the graphene/MoS2 composition for bio applications. Riyanto et al. reported that a boron-doped graphene quantum structure with MoS2 could deliver a high capacity of ~1000 mAh∙g−1.
B2O3 is a low-cost material with low environmental pollution and easy processing. It plays an important role in many applications, such as thermochemical energy storage, the addition of glass fibers, and the synthesis of boron compound materials such as BN. B2O3 is believed to enhance the electrochemical properties of MoS2 as it is conducted on 2D graphene materials[2].
Boron oxide is best suited to fully encapsulate the charge for the synthesis and growth of arsenides and phosphides. Two objectives are pursued: prevention of decomposition due to the high partial pressure of the V component and formation of a separating layer between the crucible and charge.
Synthesis method
Boron oxide, B2O3, can be prepared by burning boron at temperatures above 700 °C. It can also be prepared by dehydrating orthoboric acid, which is written either as H3BO3 or as B(OH)3:
2BOH3→B2O3+3H2O
B2O3 is a glassy white solid that will dissolve in hot water to reform the acid.
Orthoboric acid, also called boracic or boric acid, is usually made by precipitation from a borax solution on treatment with sulfuric acid. The acid is a solid at room temperature and is only sparingly soluble in water. Dilute aqueous solutions are often used as mild antiseptics. Orthoboric acid is a very weak Lewis acid that, in reaction with water, produces hydronium ions as follows:
BOH3+2H2O→BOH4−+H3O+
B(OH)3 is the product of the hydrolysis of numerous boron compounds:
B2H6+6H2O→2BOH3+6H2
BX3+3H2O→BOH3+3HXX=Cl,Br,I
Reference
[1] D. Gokdai, T. Toḡrul, M. Gürü. “A new approach to the manufacturing of elemental boron from boron oxide by carbon monoxide.” International Journal of Hydrogen Energy 42 1 (2017): 18028–18033.
[2] Thang Phan Nguyen, Il Tae Kim. “Boron Oxide Enhancing Stability of MoS2 Anode Materials for Lithium-Ion Batteries.” ACS Applied Electronic Materials (2022).
Related articles And Qustion
Lastest Price from Boron oxide manufacturers

US $1.00-4.00/KG2025-09-11
- CAS:
- 1303-86-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG
US $10.00/KG2025-04-21
- CAS:
- 1303-86-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt