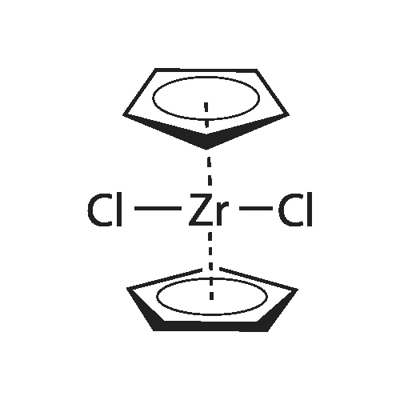
Bis(cyclopentadienyl) zirconium dichloride catalyzed acetylation of phenols, alcohols and amines
1.Introduction
Acylation of aromatic alcohols, phenols, and amines is an important reaction in organic synthesis and is usually performed employing acid anhydrides or acyl chlorides in the presence of stoichiometric amount of amine bases, such as tertiary amines, tributylphosphine and 4-(dimethylamino) pyridine or 4-pyrrolidinopyridine. On the other hand, protic acids, such as p-toluenesulfonic acid and Lewis acids, such as zinc chloride, cobalt chloride, scandium trifluoromethanesulfonate, trimethylsilyl trifluoromethanesulfonate are well known to catalyze the acylation reactions. Recently, montmorillonites, perchloric acid adsorbed on silica gel, aluminium dodecatungstophosphate, Sc(NTf2)3, Zeolites, TaCl5, Nafion-H, Yttria-zirconia, InCl3, Bi(OTf)3, HBF4–SiO2, Mg(ClO4)2, BiOClO4, and ZrCl4 have also been utilized to achieve the acylation of alcohols, phenols, thiols, and amines.
The major drawbacks in some of the above described protocols include harsh reaction conditions, use of hazardous materials, use of excess acetylating agent, scope for potential side reactions with acid-sensitive substrates and in most of the cases being applicable to alcohols only, e.g. pyridine derivatives, such as DMAP and 4-pyrrolidinopyridine are highly toxic; Bu3P is flammable and air sensitive; metal triflates lead to potential side reactions (rearrangement, dehydration etc.) with acid-sensitive substrates necessitating the use of a large excess of Ac2O and low temperature; use of costly catalyst, special efforts required to prepare the catalyst (triflate and aluminium dodecatungstophosphate) and explosive property (perchloric acid and its salt).
Recently, ZrCl4 has also been utilized to achieve the acetylation but it is highly corrosive, hygroscopic and difficult in handling. The natural abundance and easy availability of zirconium makes Zr(IV) compounds less expensive. Thus, bis(cyclopentadienyl)zirconium dichloride has been regarded as one of the stable non-hazardous organometallic compounds and ideal catalyst for catalytic applications as evidenced by its applications in polymerisation. In our earlier report, we have shown the use of bis(cyclopentadienyl)zirconium dichloride as an efficient catalyst for the ring opening of epoxides with alcohols and in the synthesis of bis(indolyl) methanes and alkylation of heteroaromatics. In continuation of our work on zirconocene catalyzed reactions, herein, we report Cp2ZrCl2 catalyzed acetylation of phenols, alcohols and amines with acetic anhydride as an acetylating agent.
2.Experimental
The catalyst bis(cyclopentadienyl)zirconium dichloride was synthesized following the reported literature procedure. All substrates are commercially available and used without further purification. In a typical procedure, 2-naphthol (360;mg, 2.5mmol) was reacted with acetic anhydride (0.24ml, 2.5mmol) in the presence of Cp2ZrCl2 catalyst (7mg, 0.96mol% based on substrate) for the required period of time at room temperature under stirring. After completion of the reaction monitored by TLC, the mixture was subjected to flash-chromatography using hexane as eluent to afford 2-naphthyl acetate as pure product (415mg, 89% Yield). The isolated yields are calculated on the basis of the weight of the pure product obtained. (In some cases, the weight balance is cross-checked with the left over quantity of the unreacted substrate recovered from the column chromatography). In all the reactions, we observed only the corresponding desired product and no byproduct. The products are characterized on the basis of1H NMR and mass.1H NMR: (CDCl3) δ 2.36(s, 3H), 7.24 (d,J = 8.62 Hz, 1H), 7.47(m, 2H), 7.56(s,1H), 7.8(m, 3H) further confirmed by Mass data: EIMS (m/z) 186 (M+), 144(100), 115(45).
3.Results and discussion
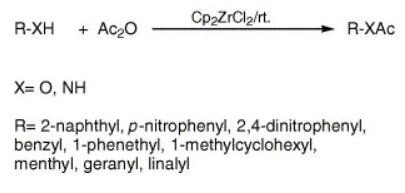
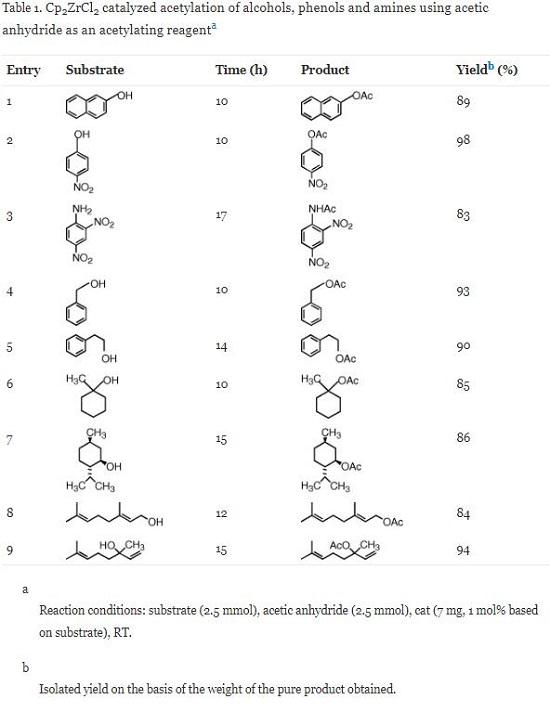
The results of the acetylation of phenols, alcohols and amines using Cp2ZrCl2 catalyst (Scheme 1) are shown in Table 1, which amply demonstrates the generality and scope of the reaction with regard to structurally diverse phenols, alcohols and amines. Thus, the treatment of the substrate with acetic anhydride in presence of 1 mol% catalyst based on substrate under solvent free conditions at room temperature provided the corresponding acetate in excellent yields (86–98%). The described methodology illustrates a very simple acetylation procedure, which has wide applicability extending the scope to benzylic, primary, secondary, tertiary, allylic and cyclic alcohols. Sterically hindered and electron deficient phenols and amines are efficiently acetylated under similar conditions and solvent free condition. It was notable that the position of the substituents (entry 2 and 3) showed no significant effect on the conversion of the reaction, which indicated that the catalyst system exhibited the tolerance for substituents. Similar observations were also reported by Moghadham et al. Secondary and tertiary alcohols do not experience any competitive dehydration (entries 6, 7 and 9) and no rearrangement took place for the allylic substrate (entry 9). Optically active menthol is acetylated without any racemization (observed [α]D = −76; reported [α]D = −80, entry 7).
Related articles And Qustion
See also
Lastest Price from Bis(cyclopentadienyl)zirconium dichloride manufacturers

US $0.00-0.00/kg2025-10-23
- CAS:
- 1291-32-3
- Min. Order:
- 1kg
- Purity:
- ≥98.0%
- Supply Ability:
- 20 tons

US $10.00/KG2025-04-21
- CAS:
- 1291-32-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt