Biosynthesis of Pyridoxal Phosphate: A Crucial Coenzyme in Biochemical Reactions
General Description
Pyridoxal phosphate is a coenzyme that acts as a catalyst in various biochemical reactions. It plays a crucial role in stabilizing the negative charge at Cα of amine-containing substrates, resulting in a range of reactions including transamination, racemization, decarboxylation, substitution, and α, β, and γ elimination reactions of amino acids. Pyridoxal phosphate is synthesized through the DXP-dependent pathway in E. coli and the novel R5P-dependent pathway in fungi. Understanding the biosynthesis of Pyridoxal phosphate and its cofactors can lead to the development of new therapeutics and industrial applications. This coenzyme is essential for maintaining overall health and wellbeing.

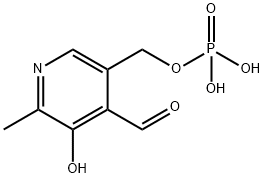
Figure 1. Pyridoxal phosphate
A Crucial Coenzyme in Biochemical Reactions
Pyridoxal phosphate is a coenzyme that plays a crucial role in various biochemical reactions in living organisms. It is the active form of vitamin B6 and acts as a catalyst in a diverse group of enzymes such as oxidoreductases, transferases, hydrolases, lyases, and isomerases. Pyridoxal phosphate is involved in the stabilization of the negative charge at Cα of amine-containing substrates, which is the major catalytic function of this cofactor. This results in a range of reactions including transamination, racemization, decarboxylation, substitution, and α, β, and γ elimination reactions of amino acids. Furthermore, Pyridoxal phosphate participates in radical-mediated reactions, aminosugar deoxygenation, and catalysis involving the phosphate group of Pyridoxal phosphate in muscle glycogen phosphorylase b. Recent studies have also reported on vitamin B6's role as a singlet oxygen quencher in fungi. The interconversion of Pyridoxal phosphate with its six vitamers allows for flexibility in its activity. In conclusion, Pyridoxal phosphate is essential for the proper functioning of many vital metabolic pathways and plays a critical role in maintaining overall health and wellbeing. 1
Biosynthesis
DXP-dependent pathway
Pyridoxal phosphate is an important cofactor in many enzymatic reactions involved in amino acid metabolism, neurotransmitter synthesis, and other cellular processes. The ‘DXP dependent’ biosynthetic route of Pyridoxal phosphate has been well characterized in E. coli. It starts with the formation of DXP from pyruvate and glyceraldehyde 3 phosphate in a thiamin pyrophosphate-dependent reaction. DeoxyD-xylulose 5-phosphate synthase (DXS) plays an important role in isoprenoid biosynthesis and is a target of interest for antibiotic, antimalarial, and herbicide development. The three carbon substrate 3-hydroxy-1-aminoacetone phosphate is synthesized from D-erythrose 4-phosphate in four steps involving various enzymes such as erythrose 4-phosphate dehydrogenase and PdxA. PNP synthase catalyzes the condensation of DXP with 3-hydroxy-1-aminoacetone phosphate to form PNP, which is oxidized by PNP oxidase, PdxH, to form Pyridoxal phosphate. The X-ray crystal structures of various enzymes involved in this biosynthetic route have been determined, and their biochemical characterization explains the preference for certain substrates and cofactors. Understanding the biosynthesis of Pyridoxal phosphate and its cofactors can lead to the development of new therapeutics and industrial applications. 2
R5P-dependent pathway
In addition to the well-known DXP-dependent pathway, there is a novel ‘R5P-dependent’ pathway for the biosynthesis of Pyridoxal phosphate. The discovery of this pathway was made by studying the singlet oxygen resistance gene (SOR1) in the fungus Cercospora nicotianae, which was found to be essential for pyridoxine biosynthesis. Further research showed that SOR1, later renamed Pdx1, in complex with Pdx2, catalyzes the condensation of D-ribose 5-phosphate (R5P), glutamine, and glyceraldehyde 3-phosphate to form Pyridoxal phosphate. The structure of Pyridoxal phosphate synthase from Bacillus subtilis shows twelve Pdx1 and twelve Pdx2 subunits forming a cog wheel like structure. Pdx2 catalyzes the hydrolysis of glutamine using a Glu-His-Cys catalytic triad to produce ammonia, which diffuses through a hydrophobic channel to the active site of Pdx1 where Pyridoxal phosphate is synthesized from R5P and glyceraldehyde 3-phosphate. The equilibrium dissociation constant is reduced in the presence of glutamine. The Pyridoxal phosphate biosynthetic pathway was successfully reconstituted using the B. subtilis enzymes and R5P, glutamine, and glyceraldehyde 3-phosphate as substrates. The current mechanistic proposal for the reaction involves migration of Lys 81 from C1 to C5, and the biochemical characterization of the chromophoric intermediate was made possible by the trapping of it with tris(2-carboxyethyl)phosphine. 3
Reference
1. Mukherjee T, Hanes J, Tews I, Ealick SE, Begley TP. Pyridoxal phosphate: biosynthesis and catabolism. Biochim Biophys Acta. 2011;1814(11):1585-1596.
2. Kuzuyama T, Takagi M, Takahashi S, Seto H. Cloning and characterization of 1-deoxy-D-xylulose 5-phosphate synthase from Streptomyces sp. Strain CL190, which uses both the mevalonate and nonmevalonate pathways for isopentenyl diphosphate biosynthesis. J Bacteriol. 2000;182(4):891-897.
3. Ehrenshaft M, Bilski P, Li MY, Chignell CF, Daub ME. A highly conserved sequence is a novel gene involved in de novo vitamin B6 biosynthesis. Proc Natl Acad Sci U S A. 1999;96(16):9374-9378.
You may like
Related articles And Qustion
Lastest Price from Pyridoxal phosphate manufacturers

US $7.00/kg2025-04-21
- CAS:
- 54-47-7
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000

US $500.00-300.00/kg2025-04-21
- CAS:
- 54-47-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000