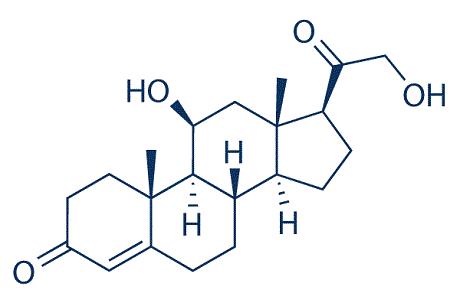
Biological functions and synthesis of 18-hydroxycorticosterone
Structural
Corticosterone is converted to aldosterone, with the synthesis of 18-hydroxycorticosterone as the next-tolast step in the biosynthesis of this mineralocorticoid. The terminal steps in the biosynthesis of aldosterone from corticosterone in the human adrenal cortex are mediated by the enzymes CYP11B1 (11β-hydroxylase)and CYP11B2 (aldosterone synthase). CYP11B1 catalyzes the 11β-hydroxylation of 11-deoxycorticosterone to corticosterone in both the zona glomerulosa and the zona fasciculata/reticularis. CYP11B2 is expressed exclusively in the zona glomerulosa, where it catalyzes the 11β-hydroxylation of 11-deoxycorticosterone to corticosterone. Then, 18-hydroxylase (CYP11B2) acts on corticosterone to form 18-hydroxycorticosterone. 18-hydroxycorticosterone is secreted and converted by 18-hydroxydehydrogenase (CYP11B2) to its metabolite aldosterone, the physiological mineralocorticoid.

Synthesis and release
Gene, mRNA, and precursor
Human CYP11B2, which catalyzes the synthesis of 18-hydroxycorticosterone, is located on chromosome 8 (8q21-q22), and consists of nine exons. Human CYP11B2 mRNA has 2935 bp, which encodes a protein of 503 residues. Tissue and plasma concentrations Recumbent position: Adult male: 10.3± 4.2 ng/ 100mL, Adult female: 12.4± 4.5 ng/100mL.There is no difference between the sexes or the periods of the menstrual cycle. Further, adrenocorticotropic hormone (ACTH) stimulation increases the plasma concentration of 18-OHB while dexamethasone markedly decreases this steroid.
Biological functions
Physiological actions Very little is known about the physiological functions of 18-hydroxycorticosterone in vertebrates. 18-hydroxycorticosterone is an intermediate precursor in the aldosterone biosynthetic pathway and 18-hydroxycorticosterone has low affinity for the MR.
18-hydroxycorticosterone binds poorly (0.2%compared to aldosterone) to renal MRs, and lacks salt-retaining action in humans. In adrenalectomized rats, the physiological dose of 18-hydroxycorticosterone has been found to have positive effects on a brush border Na+ /K+ exchanger such as corticosterone.A high concentration of 18-hydroxycorticosterone has an ability to stimulate electrogenic Na+ absorption, although its calculated Km value is outside the physiological range for corticosteroids.
Clinical implication
In patients with Cushing’s syndrome, the plasma level of 18-hydroxycorticosterone is high in conditions of adrenocortical hyperplasia and adrenocortical adenoma, and 18-hydroxycorticosterone also increases in primary aldosteronism, idiopathic hyperaldosteronism, and congenital 17α-hydroxylase deficiency.
Also, 18-hydroxycorticosterone decreases in Addison’s disease and the salt-losing type of congenital 21α-hydroxylase deficiency.Congenital hypoaldosteronism is subdivided into two types according to the levels of aldosterone and its precursors: one type shows elevated serum levels of corticosterone and low levels of 18-hydroxycorticosterone and aldosterone while the other type shows high levels of 18-hydroxycorticosterone.
The former type is generated by the loss of both 18-hydroxylation and 19-oxidation enzyme activities, and the latter type is associated with deletion of the ability to convert 18-hydroxycorticosterone to aldosterone. Further, Witzgall et al.Reported that the increase of 18-hydroxycorticosterone was significantly greater due to the administration of furosemide in patients with low-renin essential hypertension than in patients with normal renin essential hypertension, suggesting that this steroid seems to be a good marker for this phenomenon.