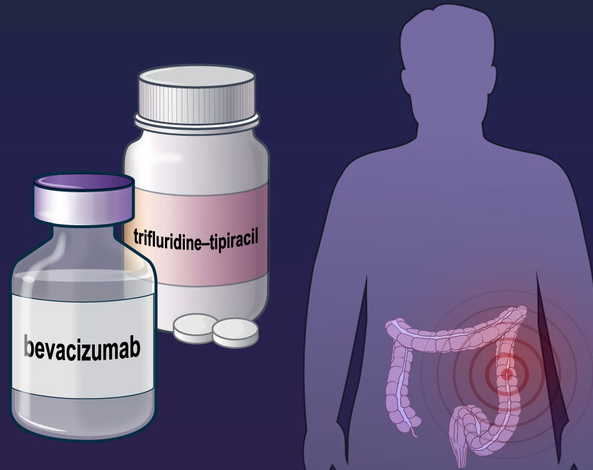
Bevacizumab: Introduction, uses, and Side effects
Introduction
Bevacizumab is an anti-angiogenic agent rationally designed to target vascular endothelial growth factor (VEGF), a key mediator in angiogenesis. The FDA approved it in February 2004 for the first-line treatment of metastatic colorectal cancer in combination with 5-fluorouracil-based chemotherapy, and it is the first approved agent to target angiogenesis.
Use
Bevacizumab is approved to be used alone or with other drugs to treat:
Cervical cancer that has not gotten better with other treatments has spread to different parts of the body or has come back. It is used with paclitaxel and either cisplatin or topotecan hydrochloride. This use is approved for the Alymsys, Avastin, Mvasi, and Zirabev brands of bevacizumab.
Colorectal cancer that has spread to other parts of the body. It is used:
With fluorouracil as the first or second treatment, With fluoropyrimidine and either irinotecan hydrochloride or oxaliplatin as the second treatment in patients whose disease has gotten worse after therapy that included bevacizumab. This use is approved for the Alymsys, Avastin, Mvasi, and Zirabev brands of bevacizumab.
Hepatocellular carcinoma (a type of liver cancer) that has spread to other parts of the body or cannot be removed by surgery. It is used with atezolizumab in patients who have not received systemic therapy. This use is approved for the Avastin brand of bevacizumab.
Glioblastoma (a type of brain cancer) that has come back. It is used in adults. This use is approved for the Alymsys, Avastin, Mvasi, and Zirabev brands of bevacizumab.
Ovarian epithelial, fallopian tube, or primary peritoneal cancer. It is used With carboplatin and paclitaxel and then alone in patients with stage III, stage IV, or recurrent cancer. It is given after surgery. This use is approved for the Avastin, Mvasi, and Zirabev brands of bevacizumab. With paclitaxel, doxorubicin hydrochloride liposome, or topotecan hydrochloride in patients whose cancer has come back, does not respond to platinum chemotherapy and has been treated with up to two types of chemotherapy. This use is approved for the Alymsys, Avastin, Mvasi, and Zirabev brands of bevacizumab, With carboplatin and paclitaxel or carboplatin and gemcitabine hydrochloride and then alone in patients whose cancer responds to platinum chemotherapy and has come back. This use is approved for the Avastin, Mvasi, and Zirabev brands of bevacizumab.
Nonsquamous non-small cell lung cancer that has spread cannot be removed by surgery or has come back. It is used with carboplatin and paclitaxel as the first therapy. This use is approved for the Alymsys, Avastin, Mvasi, and Zirabev brands of bevacizumab.
Renal cell carcinoma (a type of kidney cancer) has spread to other parts of the body. It is used with interferon-alpha. This use is approved for the Alymsys, Avastin, Mvasi, and Zirabev brands of bevacizumab.
Bevacizumab is also being studied in the treatment of other types of cancer.
Side effects
The principal adverse events seen in clinical trials are hypertension, proteinuria, arterial thrombosis, effects on wound healing, bleeding, and gastrointestinal perforation. These events are, for the most part, mild to moderate, intense, and clinically manageable (hypertension, proteinuria, minor bleeding) or are uncommon (wound healing complications, gastrointestinal perforation, and arterial thrombosis). The adverse effects profile of bevacizumab makes it a suitable adjunct to standard chemotherapy, and it is now approved for use in the USA, the European Union, and other markets worldwide. Hypertension has been associated with bevacizumab. Close monitoring is essential, especially in patients at greater risk of adverse events.
You may like
See also
Lastest Price from Bevacizumab manufacturers

US $0.00/G2025-04-21
- CAS:
- 216974-75-3
- Min. Order:
- 1G
- Purity:
- 98%min
- Supply Ability:
- 30kg/month