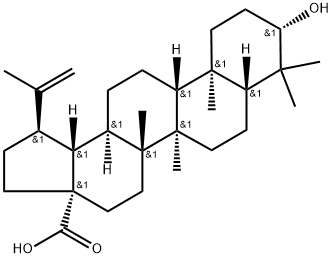
Betulinic acid - Application, Pharmacokinetics, Synthesis etc.
Clindamycin Phosphate is the phosphate salt form of clindamycin, a semi-synthetic, chlorinated Betulinic acid is a pentacyclic triterpenoid that is lupane having a double bond at position 20(29) as well as 3beta-hydroxy and 28-carboxy substituents. It is found in the bark and other plant parts of several species of plants including Syzygium claviflorum. It exhibits anti-HIV, antimalarial, antineoplastic and anti-inflammatory properties. It has a role as an EC 5.99.1.3 [DNA topoisomerase (ATP-hydrolysing)] inhibitor, an anti-HIV agent, an antimalarial, an anti-inflammatory agent, an antineoplastic agent and a plant metabolite. It is a pentacyclic triterpenoid and a hydroxy monocarboxylic acid. It derives from a hydride of a lupane. Betulinic acid is a pentacyclic triterpenoid that is lupane having a double bond at position 20(29) as well as 3beta-hydroxy and 28-carboxy substituents. It is a naturally occurring pentacyclic lupane-type triterpenoid usually isolated from birch trees, but present in many other botanical sources. It is found in different plant organs, both as a free aglycon and as glycosyl derivatives. A wide range of pharmacological activities has been described for this triterpenoid, including antiviral and antitumor effects. In addition, several other interesting properties have been identified in the fields of immunity and metabolism, namely anti-diabetic, antihyperlipidemic, and anti-inflammatory activities.

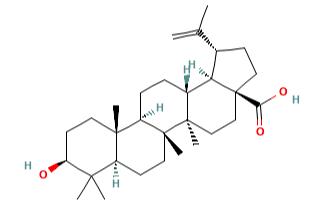
Figure 1 the molecular formula of 2,2-Dimethyl-1,3-dioxane-4,6-dione
Application and pharmacokinetics
These latter three properties make betulinic acid a highly interesting prospect for treating metabolic syndrome. The present review focuses on the therapeutic potential of this agent, along with several of its semisynthetic derivatives, which could open new frontiers in the use of natural product-based medicines.
The hybrid supposedly not only has the properties of its components, but may also have new or enhanced properties generated by the components’ synergistic coaction. In our previous studies, we have shown that the natural products are more efficient when they are hybridized. In the present study, the natural products betulin (1), betulinic acid (2) and artesunic acid (3) were chosen due to their efficient therapeutic properties.
Betulinic acid (50 mg/L, administered in drinking water for 15 days) decreased total cholesterol and triglycerides in high-fat diet-induced obese mice. The triterpene also decreased body weight, abdominal fat accumulation, blood glucose, plasma tri-glycerides, and total cholesterol. The results showed significant increases of insulin and leptin (anorexigenic hormone) in plasma, whereas the level of ghrelin (orexigenic hormone) decreased.These effects explain the reduced appetite regulation observed in high-fat diet-induced obese mice. Betulinic acid was shown to cause a greater decrease in plasma amylase activity than in that of lipase, but the results of studies conducted by Jang et al. and Kim et al. indicated that betulinic acid inhibits porcine pancreatic lipase (IC50= 21.1 µM) and has a lipolytic effect mediated by cAMP-dependent phosphodiesterase inhibition, which could lead to the observed reduction of lipid absorption in the small intestine and the increase of fat mobilization. Betulinic acid also reduces cholesterol absorption in the intestine via inhibition of hACAT. Indeed, the triterpene had an inhibitory effect on hACAT-1 (responsible for foam cell formation in macrophages) and hACAT-2 (responsible for the cholesterol absorption process in intestinal mucosal cells), with IC50values of 16.2 and 28.8 µM, respectively. In addition to its lipolytic effect in adipose tissues, betulinic acid reduced lipogenesis and lipid accumulation in various experimental models, both in vitro (HepG2 cells) and in vivo (ICR mice and Wistar rats). In the HepG2 cell assays, the following effects were demonstrated: suppression of intracellular lipid accumulation via modulation of lipogenic and lipolytic factors, inhibition of hepatic lipid accumulation via activation of the AMPK signaling pathway, increase of CAMKK expression, downregulation of the mTOR, and protein expression of S6 kinase. Ex vivo and in vivo assays showed the following effects: inhibition of SREBP1 activity and expression via modulation of a CAMKK–AMPK–mTOR–S6 kinase pathway in primary rat hepatocytes, suppression of hepatic triglyceride accumulation via modulation of a CAMKK–AMPK–SREBP1 signaling pathway in the livers of ICR mice (high-fat diet), and suppression of triglyceridemia in ICR mice fed with a high-fat diet. Activation of AMPK suppresses mRNA expression and nuclear translocation of SREBP-1 while positively regulating fatty acid oxidation through the activation of the PPAR-α and the PPARγ coactivator (PGC)-1.
These effects make sense because human PPARα is expressed in several metabolically active tissues and plays a critical role in the regulation of cellular uptake, activation, and oxidation of fatty acids. For its part, PGC-1 is expressed at high levels in both heart and skeletal muscle. Its expression decreases in situations associated with mitochondrial dysfunction, such as diabetes, suggesting that decreases in PGC-1 activity may contribute to this pathology. In this context, betulinic acid may be able to regulate lipid homeostasis. Betulinic acid also inhibited diacylglycerol acyltransferase in rat liver microsomes (IC50= 9.6 µM) in a noncompetitive manner, while also inhibiting triacylglycerol synthesis in HepG2 cells.Taken together, these results indicate that betulinic acid may help reduce hepatic lipid accumulation by modulating the AMPK–SREBP signaling pathway, which would explain its hepatic antihyperlipidemic activity. Several relevant aspects of the modification of lipid metabolism are summarized in Figure 2[1]

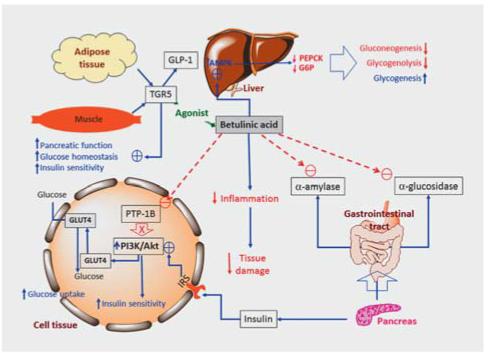
Figure 2 Effect of betulinic acid on glucose metabolism. Akt, protein kinase B; AMPK, AMP-activated protein kinase; G6P, glucose-6-phosphate; GLP-1, glucagon-like peptide 1; GLUT-4, glucose trans-porter-4; IRS, insulin receptor substrate; PEPCK, phosphoenolpyruvate carboxykinase; PI3KB, phosphatidylinositol 3-kinase-depen-dent B; PTP1B, protein tyrosine phosphatase-1B; TGR5; bile acid membrane receptor.
Synthesis
Mono- and di-esterified betulin-ferrocene hybrids 6 and 7 were obtained in 55% and 78 % yields, respectively. For the di-esterification of betulin, the reaction mixture needed to be stirred for 5-6 days whereas mono-esterification was completed after overnight stirring. The crystals of hybrid 6 were obtained by vapour diffusion crystallization in CH2Cl2 (solvent) and hexane (antisolvent)(Fig. 3). Hybrid 6 was further used for a hybridization reaction to obtain artesunic acid-betulin-ferrocene hybrid 8. The ester bond was formed between the free alcohol group of betulin-ferrocene hybrid 6 and artesunic acid in 60% yield. Besides hybridization, dimerization of natural products by nature or synthetically has been also reported as highly potent approach towards bioactive agents. Thus, in the present study, synthesis and biological evaluation of new dimers of betulin and betulinic acid were carried out as well. Ferrocene dicarboxylic chloride (14) was employed as linker compound to synthesize betulinic acid dimer 9 and betulin dimer 10.

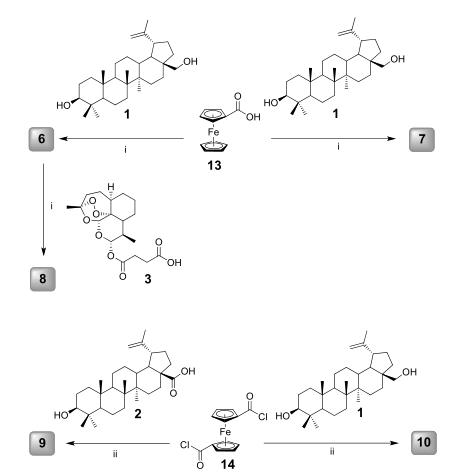
Figure 3 Synthesis of Betulinic acid(Synthetic routes of betulinic acid-/betulin-derived dimers and hybrids 6 – 12; i) DCC, DMAP, CH2Cl2, 0 ℃-rt, ii) DMAP, CH2Cl2, 0 ℃- rt.)
Toxicological and Safety
DMEM with 10% fetal bovine serum (Gibco, United States) was used for culturing. Optical density was measured on a Multiskan RC spectrophotometer (LabSystems). The MTT test was carried out according to the standard procedure [32] using the reagent 3-(4,5-dimethylthiazol-2-yl)-2,5-diphen-yltetrazolium bromide (MTT, Sigma, Unit-ed States). Cell lines were obtained from the American Type Culture Collection: U-87 MG (ATCC number HBT-14), MCF7 (ATCC number HTB-22). Immortalized human fibroblasts used as a negative control were provided by A. Schilov (Institute of Cytology and Genetics of Siberian Branch of Russian Academy of Sciences, Novosi-birsk, Russia). The viability for DMSO (negative control) was calculated as 100%. All culture ex-periments were carried out independently three times with two repeats in the experiment, and the final result is presented as concentration, which caused 50% inhibition of cell population growth (IC50 ± SEM).
References
1
2, "Synthesis of New Betulinic acidBetulinderived Dimers and Hybrids with Potent,".
3
You may like
Related articles And Qustion
See also
Lastest Price from Betulinic acid manufacturers
US $0.00-0.00/KG2025-12-11
- CAS:
- 472-15-1
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS
US $0.00-0.00/kg2025-10-22
- CAS:
- 472-15-1
- Min. Order:
- 0.10000000149011612kg
- Purity:
- 99%
- Supply Ability:
- 20tons