Beryllium: Discovery, Physical Properties, and Chemical property
Introduction
Beryllium, Be, is the first element in the second main group of the periodic table. It is a light metal with a hexagonal-closest-packed (hcp) structure. Both finely divided metals and the compounds have serious toxic effects on the lungs. In addition to the name beryllium, derived from the mineral beryl, this element with atomic number 4 was formerly called glucinium, chiefly in France, because of the pronounced sweetish taste of its salts.
Discovery
In 1797, Vauquelin discovered beryllium oxide while analyzing varieties of beryl, including emerald and aquamarine. W¨ohler and Bussy, working independently, prepared elemental beryllium in 1828 by reduction of beryllium chloride with potassium. In 1899, Lebeau obtained beryllium crystals by electrolysis of molten sodium beryllium fluoride. Industrial production of beryllium began in 1916 in several countries. The publication in 1916 by Oesterheld of the phase diagrams of beryllium with iron, nickel, and copper led to the first significant use of beryllium in 1931, namely, as an additive, about 2 wt%, in age-hardenable copper alloys.
The demand for beryllium increased around 1950 with the development of nuclear reactors because the nuclear properties and low density of beryllium seemed to indicate that the metal would be especially suitable for nonstationary nuclear reactors of the types used in airplanes, spacecraft, ships, and submarines. However, beryllium was a disappointment because it swells and becomes brittle when exposed to the simultaneous action of radioactivity and heat. However, the increased research activity in beryllium technology associated with this period produced significant advances in beryllium's chemistry, physics, and processing technology.
Uses
Most of the beryllium produced today is used for military purposes. Greater use in the civilian sector is hindered mainly by the high price, the toxicity, and the room-temperature susceptibility to brittle metal fracture. On the other hand, beryllium oxide is finding increasing use in electrical engineering as an electrical insulator.
Physical Properties
The c-to-a ratio of hexagonal-closest-packed beryllium is very small and decreases with temperature. The existence of the body-centered-cubic high-temperature modification was disputed for many years but has now been demonstrated conclusively. The transformation point is slightly below the melting point. The sum of the heat of fusion and transformation is given as 1641 J/g, which allows a heat of transformation of 284 J/g to be estimated. However, a much higher value, ≈ 837 J/g, was determined directly.
The coefficient of linear expansion is comparatively low. The electrical resistivity between 40 and 200 K is significantly lower than that of copper or aluminum. Beryllium is diamagnetic; little work has been reported on its magnetic properties. The reflexivity of beryllium is high, particularly in the infrared region. Beryllium transmits X-rays well because of its low atomic number. Even relatively long-wave gamma rays cause beryllium to emit neutrons on account of a (γ,n) reaction. Other nuclear reactions of beryllium and its most important nuclear properties are shown below:
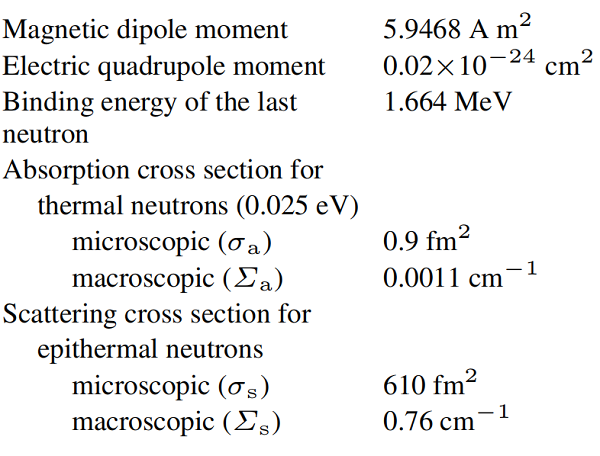
The nuclear reactions of beryllium with fast neutrons and hard γ rays do not result in long-lived radioactivity. However, additional neutrons and gaseous reaction products may be produced. In addition to the formation of noble gases and tritium, normal radiation damage also occurs as a result of elastic collisions of fast neutrons with beryllium atoms, but the effect of this radiation damage can hardly be separated from the effect of the gas formation. The moderator and reflector properties of beryllium for thermal and epithermal neutrons are also favorable.
Chemical property
The two 2s electrons of beryllium are valence electrons. The first ionization potential (Be0 → Be+ + e) is 9.32 eV, and the second (Be+ → Be2+ + e) is 18.21 eV. It is far more similar to aluminum than to magnesium or calcium. For example, aluminum is amphoteric, and in humid air and water, vapor forms a strongly adhering surface layer of oxide that prevents further oxidation up to about 600 ◦C. Above 600 ◦C, the oxide layer thickens, but oxidation that destroys the metal by breakaway does not occur below 700 ◦C. The affinity of beryllium for oxygen is very great Be (s) +1/2O2 (g) →BeO (s) ∆G0 T(J/g) = −66469+10.5T±4.7 and beryllium is an excellent reducing agent. At temperatures > 900 ℃, it reacts violently with nitrogen or ammonia to form beryllium nitride, Be3N2. However, beryllium does not react with hydrogen, even at high temperatures, because the formations of the hydrides BeH2 and BeH, which are difficult to prepare, are endothermic, and the hydrides rapidly decompose at only slightly elevated temperatures. Below 500 – 600 ◦C, beryllium is not attacked by dry carbon dioxide and is attacked only very slowly by moist carbon dioxide.
Beryllium powder reacts with fluorine at room temperature, and at elevated temperatures, it reacts with chlorine, bromine, and iodine and with sulfur, selenium, and tellurium vapor; in each case, beryllium burns with a flame. The adhering oxide film protects beryllium from cold and hot water attacks. It also protects cold beryllium from attack by oxidizing acids. The stability of the film can be increased by the addition of dichromate to the water to form a protective chromate layer, as in the case of aluminum. Beryllium dissolves in dilute nonoxidizing mineral acids, accompanied by hydrogen evolution and salt formation. In accordance with its amphoteric nature, it is also attacked by aqueous hydroxide, which is accompanied by hydrogen evolution and beryllate formation. Since most beryllium compounds have a highly exothermic heat of formation, beryllium reduces the salts and the borates and silicates of many metals. Molten alkali-metal hydroxides react explosively with beryllium. Beryllium forms several intermetallic phases with carbon, all transition metals; the alkaline earth metals magnesium, calcium, and strontium; and the metalloids boron, arsenic, tellurium, etc. Some of these phases have extensive homogeneity ranges and high melting points.
Beryllium is very stable towards liquid lithium, sodium, potassium, zinc, magnesium, cadmium, mercury, gallium, indium, tin, lead, antimony, and bismuth. Some metals do not dissolve beryllium at all, even at high temperatures, so long as oxygen is not present. If the melts contain metal oxides, the metal oxides are reduced by beryllium with the formation of beryllium oxide. The layer of BeO formed in this way is not adherent and is removed by the melt, so a fresh surface of beryllium is always available for reduction. Beryllium is very reactive in the liquid state, reacting with most oxides, nitrides, sulfides, and carbides, including those of magnesium, calcium, aluminum, titanium, and zirconium.