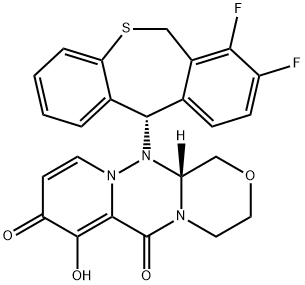
Baloxavir: pharmacodynamics, pharmacokinetics and clinical applications
General Description
Baloxavir is a potent antiviral medication targeting influenza by inhibiting the cap-dependent endonuclease, disrupting viral replication. Its active form, S-033447, exhibits strong antiviral activity against various influenza strains and maintains efficacy even when treatment is delayed. Pharmacokinetically, it undergoes rapid hydrolysis, with peak plasma concentrations achieved within 4 hours and primarily eliminated via biliary excretion. Approved for individuals aged 12 and older, baloxavir offers rapid symptom alleviation, reduced viral shedding, and convenient single-dose regimen. Its prophylactic use has shown efficacy in preventing influenza transmission, especially in high-risk populations. Overall, baloxavir presents significant potential in clinical influenza management and future antiviral strategies, particularly against strains resistant to traditional treatments.
Figure 1. Capsule of baloxavir
Pharmacodynamics
Baloxavir, as a small molecule selective inhibitor of cap-dependent endonuclease, exerts its pharmacodynamic effects by targeting a key enzyme in influenza viruses responsible for initiating mRNA synthesis. The active form of baloxavir, S-033447, has demonstrated potent antiviral activity against various influenza A and B laboratory virus strains and clinical isolates, including the NA/H274Y mutant strain. In vitro studies have shown that baloxavir can suppress viral titer to 90% at concentrations ranging from 0.46 to 0.98 nmol/L for influenza A and 2.21 to 6.48 nmol/L for influenza B. Furthermore, baloxavir exhibits significant antiviral activity against other influenza strains, such as H5N1 and H7N9 subtype avian influenza virus strains, as well as influenza A and B strains resistant to oseltamivir. Studies in mouse models have revealed that single-day dosing of baloxavir significantly improves mortality compared to oseltamivir, with a profound reduction in viral titers within 24 hours of administration. Even when treatment initiation is delayed by 24–96 hours after inoculation of a lethal quantity of influenza A virus, baloxavir maintains its efficacy. However, it's important to note that in vitro tolerance isolation tests have shown that the presence of an I38T amino acid mutation in the polymerase acidic protein region, which is the binding target site of baloxavir, can reduce susceptibility to baloxavir by 100-fold. 1
Pharmacokinetics
Baloxavir, when administered orally at a dose of 40 mg to healthy male subjects in a fasting state, exhibits unique pharmacokinetic properties. The drug undergoes rapid hydrolysis to its active form, S-033447, resulting in peak plasma concentrations (Cmax) of baloxavir that are initially undetectable. The Cmax of S-033447 is achieved within a median of 4 hours, displaying linear, dose-proportional systemic exposure across a single dose range of 6–80 mg in healthy adult subjects. S-033447, the active form of baloxavir, is highly protein-bound (93–94%) and possesses a large apparent volume of distribution. It is primarily metabolized by UGT1A3 to glucuronic acid conjugate and subsequently by CYP3A to form sulfoxide. Baloxavir, on the other hand, is primarily eliminated via biliary excretion with 80% of the drug recovered in the feces and 15% in the urine following a single dose. The estimated mean elimination half-life of S-033447 is 96 hours. Dosage adjustments based on body weight are necessary, as indicated by in vitro studies, and it has been observed that baloxavir exposure is similar when administered to both adults and pediatric patients. Furthermore, both baloxavir and S-033447 are substrates of P-glycoprotein (P-gp), with baloxavir weakly inhibiting P-gp, CY2B6, CYP2C8, and CYP3A, and S-033447 inhibiting P-gp and breast cancer receptor protein, although these interactions are not expected to be clinically relevant. Food appears to decrease baloxavir exposure, but specific recommendations regarding administration with food are not provided in the prescribing information. 2
Clinical applications
Baloxavir marboxil is a novel antiviral medication with promising clinical applications. Approved for the treatment of uncomplicated influenza in individuals aged 12 years and older, Baloxavir works by inhibiting the cap-dependent endonuclease within the influenza virus, thereby disrupting viral replication. Its clinical applications extend to both treatment and prophylaxis, offering several advantages over traditional neuraminidase inhibitors. In the clinical setting, Baloxavir has demonstrated rapid symptom alleviation and reduced viral shedding compared to placebo and oseltamivir. Additionally, its single-dose regimen presents a convenient and potentially more adherent treatment option for patients. Furthermore, Baloxavir's prophylactic use has shown efficacy in preventing household transmission of influenza, making it particularly valuable in outbreak control and high-risk populations. Although resistance to Baloxavir has been reported, its distinct mechanism of action provides an alternative treatment for influenza strains resistant to neuraminidase inhibitors. Ongoing research is exploring the potential of Baloxavir in combination therapies and its effectiveness against emerging influenza strains. In conclusion, Baloxavir showcases significant potential in the clinical management of influenza and holds promise for future antiviral strategies. 3
Reference
1. Taniguchi K, Kobayash M, Ando Y, et al Inhibitory effect of S-033188/S-033447, a novel inhibitor of influenza virus cap-dependent endonuclease, against highly pathogenic avian influenza virus A/H5N1 [abstract no. P1974 and poster]. In: 27th European congress of clinical microbiology and infectious diseases (ECCMID). 2017.
2. Shionogi & Co. Ltd. XofluxaTM (baloxavir): Japanese prescribing information 2018. http://www.pmda.go.jp/. Accessed 26 Feb 2018.
3. Heo YA. Baloxavir: First Global Approval. Drugs. 2018 Apr;78(6):693-697.
Related articles And Qustion
See also
US $0.00/kg2025-11-15
- CAS:
- 1985605-59-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $0.00-0.00/g2025-04-21
- CAS:
- 1985605-59-1
- Min. Order:
- 10g
- Purity:
- 0.99
- Supply Ability:
- 5000g