Asymmetric Silane Reductions
A very effifi cient asymmetric reduction of arylalkyl ketones has been shown. The reaction, which does not work well for prochiral dialkyl ketones, is carried out with PMHS in the presence of a chiral titanium catalyst.
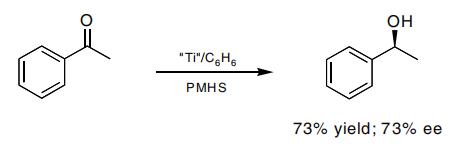
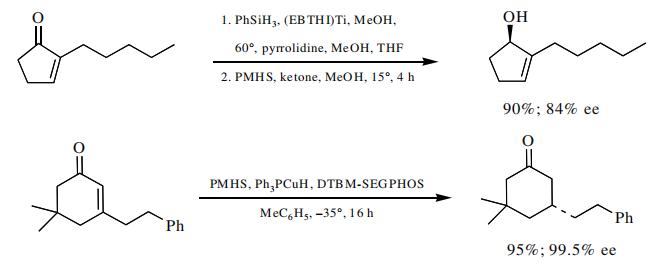
A number of asymmetric, silane-based reductions have been reported. In many cases these result in very high enantioselectivity and offer an alternative to the asymmetric hydrogenation protocol. Enones have been reduced in a 1,2-fashion, as well as in a 1,4-manner, with high ee values.
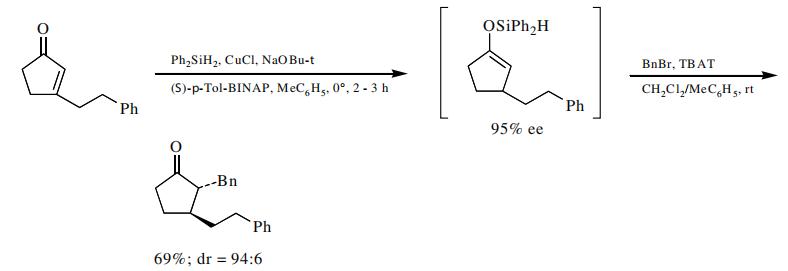
The intermediate enol silyl ether from the reduction of an enone can be trapped with benzyl bromide.
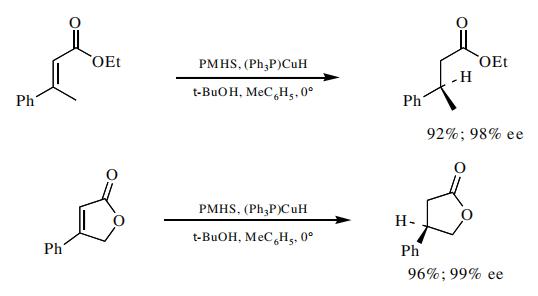
The DTBM-SEGPHOS-catalyzed PMHS reduction of α,β-unsaturated esters provides the saturated ester in high enantiomeric excess.
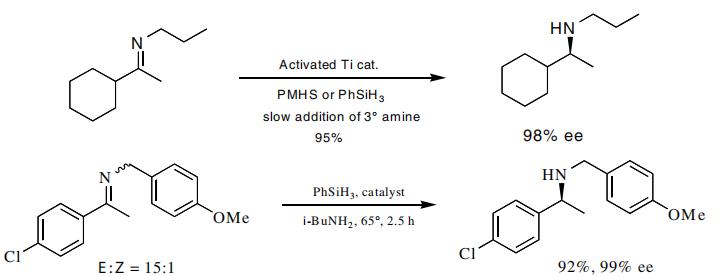
Buchwald and coworkers have reported the reduction of imines in very high enantiomeric excess through the use of a titanium catalyst activated with phenylsilane and the reduction with polymethylhydrogen siloxane or phenylsilane. The asymmetric reduction of imines has been reported in very high enantiomeric excesses.