Applications of Stevioside
Stevioside, an abundant component of Stevia rebaudiana leaf, has become well-known for its intense sweetness (250-300 times sweeter than sucrose) and is used as a non-caloric sweetener in several countries. A number of studies have suggested that, beside sweetness, stevioside along with related compounds, which include rebaudioside A (second most abundant component of S. rebaudiana leaf), steviol and isosteviol (metabolic components of stevioside) may also offer therapeutic benefits, as they have anti-hyperglycemic, anti-hypertensive, anti-inflammatory, anti-tumor, anti-diarrheal, diuretic, and immunomodulatory actions. It is of interest to note that their effects on plasma glucose level and blood pressure are only observed when these parameters are higher than normal. As steviol can interact with drug transporters, its role as a drug modulator is proposed.
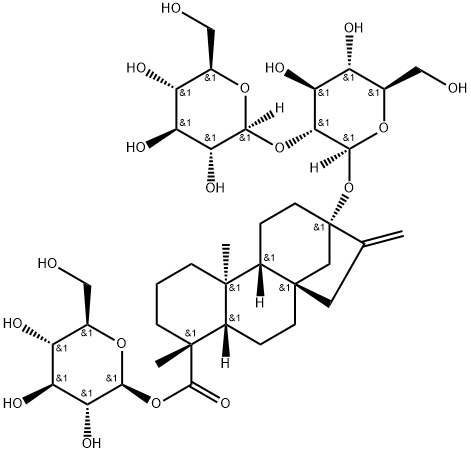
Stevioside is extracted from the leaves of Stevia rebaudiana Bertoni, a plant growing in South America and several Asian countries. Stevioside is a glycoside consisting of the aglycone steviol (ent-13-hydroxykaur-16-en-19-oic acid) and three glucose molecules. The sweetness of stevioside is accompanied by a licorice-like aftertaste.
Applications
Stevioside is a glycoside from the stevia plant. Stevioside is a natural sweetening agent with sweetness about 250 times that of sugar with negligible effect on blood glucose. Stevioside, much like ot her steviol glycoside is known for its application in treatment of many diseases like diabetes and high blood pressure. It is also used as a food additive and in dietary supplements.
Leaves of the stevia plant have been used for centuries in Brazil and Paraguay to sweeten foods and beverages. Stevioside can be used in soft drinks, Japanese-style vegetable products, table-top sweeteners, confectionery, fruit products and seafood, and in some countries as stevioside-rich Stevia extracts.
Safety and status
Stevia extracts have been approved for food use in several South American and Asian countries but lack approval in Europe and North America and on an international level. Safety studies on stevioside and Stevia rebaudiana have not been accepted internationally, owing to the lack of generally accepted specifications. In 1999, the SCF reiterated the opinion that “stevioside is not acceptable as a sweetener on the presently available data.”
The JECFA reviewed stevioside in 1998 but could not quantify an acceptable daily intake (ADI) because of inadequate data on the composition and safety of stevioside. In 2000, the European Commission refused a request for marketing authorization for Stevia rebaudiana plants and dried leaves. Stevioside, as a sweetener, is not permitted in the USA and may not be used or marketed in Europe.
You may like
Related articles And Qustion
See also
Lastest Price from Stevioside manufacturers

US $0.00/kg2025-09-18
- CAS:
- 57817-89-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS

US $0.00-0.00/kgs2025-06-28
- CAS:
- 57817-89-7
- Min. Order:
- 25kgs
- Purity:
- ≥99.0%
- Supply Ability:
- 100 tons