Applications of Benzophenone imine
Benzophenone imine(Ph2CH=NH) is an organic compound with the formula of (C6H5)2C=NH. Benzophenone imine is widely used as a reagent for the protection of primary amines, and the starting materials to synthesize aniline[1]. Benzophenone imine is available via the addition of MeOH to the complex of a nitrile and a Grignard reagent or the reaction between benzophenone and ammonia. Benzophenone imine is commonly used as an ammonia surrogate.
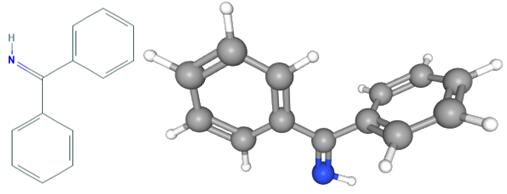
Fig 1. Chemical structure formula and three-dimensional structure of Benzophenone imine
Benzophenone imine is prepared by the addition of phenylmagnesium bromide to benzonitrile followed by hydrolysis with MeOH or by reaction of benzophenone with ammonia.In 1988, A. G. Guimanini discovered a new route to synthesize benzophenone imine via a reaction using benzophenone ammonia. A chemical pure-grade ammonia gas is added to a benzophenone solution, forming Ph2C=NH2+. After sodium hydroxide pellets are added to the solution, the Ph2C=NH2+ is neutralized, generating the expected benzophenone imine[1].
Benzophenone imine is a useful ammonia equivalent in the Buchwald–Hartwig amination and an important intermediate for the synthesis of N-protected primary amines. Benzophenone imine is utilized in the preparation of nitrile ylide dimers.
Benzophenone imine, a useful protecting group for primary amines, is introduced by reaction with the amine salt (CH2Cl2, room temperature). The resulting Schiff bases are stable to flash chromatography and have been used in conjunction with other anion-stabilizing groups, especially in the field of amino acid chemistry, to provide activation for proton abstraction. Reaction of protected derivatives with base followed by alkylation with an alkyl halide (cases a (R=H) and d), an alkynyl(phenyl)iodonium triflate (case b), or intramolecular alkylation (case c) establish new carbon-carbon bonds at the indicated center[2-5].
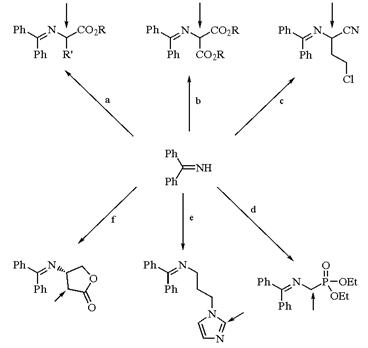
Benzophenone imine can also serve as a simple protecting group to allow acylation (case e) or formylation (case f) at a remote site. Removal is accomplished with either mild acid or catalytic hydrogenolysis[6].
Primary amines can be protected benzophenone imine, and the protected amines are stable in flash chromatography.
Buchwald-Hartwig amination is a very important kind of reaction for coupling aromatic halide and amine to form carbon-nitrogen bonds with the help of palladium-involved catalysts. In order to obtain anilines, ammonia is required in this reaction. However, ammonia can bind to palladium tightly, which makes the normal Buchwald-Hartwig reaction unavailable. In 1997, Buchwald et. al found that benzophenone imine can be used as an ammonia-equivalent and solve the above limitations[7].
References
[1] Verardo, G.; Giumanini, A. G.; Strazzolini, P.; Poiana, M. (1988). "Ketimines From Ketones and Ammonia". Synthetic Communications. 18 (13): 1501–1511.
[2] O'Donnell, M. J.; Polt, R. L. JOC 1982, 47, 2663.
[3] Beak, P.; Zajdel, W. J.; Reitz, D. B. CRV 1984, 84, 471.
[4] O'Donnell, M. J.; Bennett, W. D.; Wu, S. JACS 1989, 111, 2353.
[5] Genet, J.-P.; Uziel, J.; Touzin, A. M.; Juge, S. S 1990, 41.
[6] Hoekstra, W. J.; Press, J. B. H 1993, 35, 89.
You may like
Related articles And Qustion
Lastest Price from Benzophenone imine manufacturers
US $0.00-0.00/kg2025-11-20
- CAS:
- 1013-88-3
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 100tons

US $0.00-0.00/KG2025-04-15
- CAS:
- 1013-88-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg