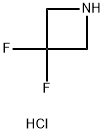
Applications of 3,3-Difluoroazetidine Hydrochloride
3,3-Difluoroazetidine hydrochloride(C3H6ClF2N) is colorless to yellow solid. 3,3-Difluoroazetidine hydrochloride can be considered as a fairly typical cyclic amine. Strain in the four-membered ring is less than that in the three-membered aziridine system, and as a result azetidines show few, if any, of the exceptional properties associated with aziridines. Thus, ring cleavage reactions occur with greater ease than in larger ring cyclic amines, but much less readily than with aziridine; for example, unlike aziridines, azetidines do not function as alkylating agents. The ring is generally thermally stable and is unreactive towards reducing agents. Although ring cleavage by nucleophiles occurs, the fact that many azetidines are prepared under basic conditions indicates that the ring strain does not make them especially labile. This means that a wide variety of transformations can be performed on attached functional groups without effect on the azetidine ring.

Fig 1. Chemical structure formula and three-dimensional structure
3,3-difluoroazetidine hydrochloride is a useful starting material and useful building block for the synthesis of various pharmaceutically active molecules, such as PI3K inhibitors, CB2 Receptor agonists, Thrombin inhibitors and bromodomain inhibitors.
(1) Novel acetylcholine and carbamoylcholine analogues: Development of a functionally selective α4β2 nicotinic acetylcholine receptor agonist[1].
(2) Tetrahydrochromenoimidazoles as potassium-competitive acid blockers (P-CABs): Structure-activity relationship of their antisecretory properties and their affinity toward the hERG channel[2].
(3) Spiroindane based amides as potent and selective MC4R agonists for the treatment of obesity[3].
(4) Studies of CDK 8/19 inhibitors: Discovery of novel and selective CDK8/19 dual inhibitors and elimination of their CYP3A4 time-dependent inhibition potential[4].
(5) In Vivo and Mechanistic Studies on Antitumor Lead 7-Methoxy-4-(2-methylquinazolin-4-yl)-3,4-dihydroquinoxalin-2(1H)-one and Its Modification as a Novel Class of Tubulin-Binding Tumor-Vascular Disrupting Agents. Design, synthesis and pharmacological evaluation of new acyl sulfonamides as potent and selective Bcl-2 inhibitors.
References
[1] C.P.Hansen, et al, J. Med. Chem., 2008, 51(23), pp 7380-7395.
[2] A.M.Palmer, et al, J. Med. Chem., 2010, 53(9), pp 3645-3674.
[3] S.He, et al, Bioorg. Med. Chem. Lett., 2010, 20(15), pp 4399-4405.
[4] J.Fujimoto, et al, Bioorg. Med. Chem. Lett., 2017, 25(12), pp 3018-3033.
You may like
See also
Lastest Price from 3,3-DIFLUOROAZETIDINE HYDROCHLORIDE manufacturers
US $0.00-0.00/kg2025-04-04
- CAS:
- 288315-03-7
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton
US $0.00/kg2025-03-03
- CAS:
- 288315-03-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS