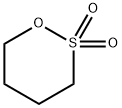
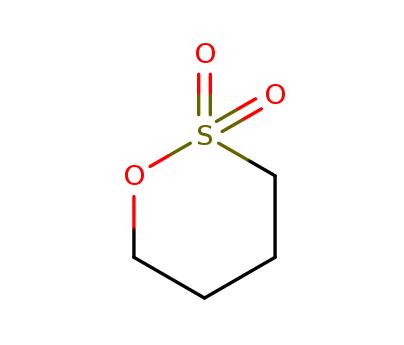
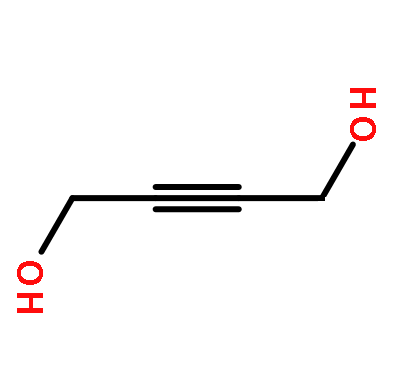
Applications of 1,4-Butane sultone
Applications of 1,4-Butane sultone
Butane sultone integrated superhydrophilic polyamide membranes for efficient ionic separation
Organically modified polyamide (PA) selective layer, with ultrahigh perm-selectivity good interfacial compatibility with porous support ion-sieving membranes, has great importance in advanced separation ions. Herein, we report synthesis 1,4-butane sultone (BS) integrated polyamide“BS@PA”selective layer via conventional interfacial polymerization (IP). The grafted BS makes BS@PA selective layer superhydrophilic highly negatively charged, ultimately increases membrane water/salt separation properties. While osmotic-driven ionic-separation, BS@PA membrane exhibits a high water permeability 36.9 79.8 mol·m−2·h−1·bar−1 with NaCl MgCl2 salts reverse permeation rates 6.5×10−3 1.8×10−3 mol·m−2·h−1·bar−1, respectively. As compared to blank PA membrane (control membrane), BS@PA membrane demonstrates higher water permeation rates with extremely low salt/ion permeation leakage, exhibiting upper-bound water/salt, ion selectivity. The outperformed ion-separation BS@PA membrane is ascribed to superhydrophilic highly negatively charged PA selective layer.
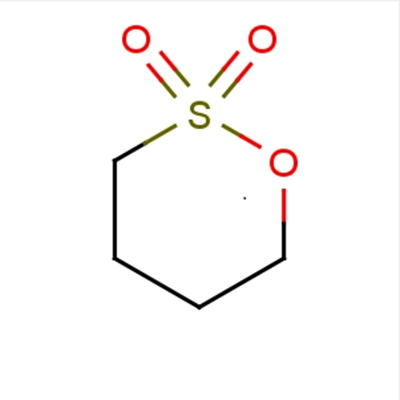
1,4-Butane sultone and New telechelic poly(butylene terephthalate) (PBT) ionomers
New telechelic poly(butylene terephthalate) (PBT) ionomers, characterized by presence some aliphatic sulfonated chain ends, were prepared by reaction with 1,4-butane sultone. Two methods synthesis were compared final polymers, containing different percentages ionic groups, were characterized, with particular attention to thermal properties. Thermogravimetric analysis shows that presence ionic groups does not modify thermal stability PBT. The differential scanning calorimetry indicates that crystalline phase crystallinity degree PBT do not change in presence ionic chain ends, whereas crystallization rate was found to be strongly modified. The ionomers crystallize at very high rate, due to nucleating effect ionic aggregations formed in melt.
1, 4-Butane sultone is used to synthesize various quinazoline derivatives
1,4-Butane sultone functionalized graphitic carbon nitride nanosheets (g-C 3 N 4 @Bu-SO 3 H) was prepared applied as an efficient heterogeneous catalyst for synthesis various quina-zolines derivatives with high yield. In next step, structure morphology catalyst was characterized by different analyses such as, FT-IR, EDS, XRD FE-SEM. On other side, considering noticeable features g-C 3 N 4 @Bu-SO 3 H such as high stability, easy to synthesize, non-toxicity, excellent reusability, so on, synthesis 2,3-dihydroquinazolines derivatives with numerous advantages such as short reaction time reaction condition, easy separation etc were realized.
References:
[1] GHAFURI H, NASRI Z, TAJIK Z. 1,4-Butane-Sultone Functionalized Graphitic Carbon Nitride as a Highly Efficient Heterogeneous Catalyst for the Synthesis of 2,3-Dihydroquinazolines Derivatives[J]. The 26th International Electronic Conference on Synthetic Organic Chemistry, 2022, 19 1: 0. DOI:10.3390/ecsoc-26-13672.
[2] C. BERTI. Modification of poly(butylene terephthalate) by reaction with 1,4-butane sultone; synthesis and thermal characterization of new telechelic PBT ionomers[J]. e-Polymers, 2008, 8 1. DOI:10.1515/epoly.2008.8.1.865.
[3] SHABAB HUSSAIN, Xinsheng P, Zhizhen Ye. Butane sultone integrated superhydrophilic polyamide membranes for efficient ionic separation[J]. Desalination, 2023, 558: Article 116611. DOI:10.1016/j.desal.2023.116611.
Related articles And Qustion
See also
Lastest Price from 1,4-Butane sultone manufacturers

US $10.00/KG2025-04-21
- CAS:
- 1633-83-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5tons

US $99.00-66.00/kg2025-04-21
- CAS:
- 1633-83-6
- Min. Order:
- 0.0010000000474974513kg
- Purity:
- 99%
- Supply Ability:
- 5000