Application researchers of 4-cyanopyridine
Introduction
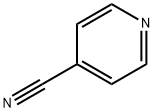
4-Cyanopyridine (Figure 1) is an important intermediate that can be used in the agricultural and pharmaceutical industries for the production of composite pyridine derivatives, as well as for the production of isoniazid, isoniazid, and remifentanil. This article mainly summarizes other application studies of 4-cyanopyridine.

As versatile spectroscopic probe for cytochrome P450 BM3
This paper reports the development of two assays, involving the use of a heme ligand, 4-cyanopyridine, which provides a route to the quick and easy determination of substrate dissociation constants for reduced P450 BM3, and presents a means of estimating heme reduction potentials across a series of closely related BM3 mutants without the requirement for redox potentiometry.[1]
The nitrogenous π-acceptor ligand 4-cyanopyridine (4CNPy) exhibits reversible ligation to ferrous heme in the flavocytochrome P450 BM3 (Kd=1.8μm for wild type P450 BM3) via its pyridine ring nitrogen. The reduced P450-4-cyanopyridine adduct displays unusual spectral properties that provide a useful spectroscopic handle to probe particular aspects of this P450. 4-Cyanopyridine is competitively displaced upon substrate binding, allowing a convenient route to the determination of substrate dissociation constants for ferrous P450 highlighting an increase in P450 substrate affinity on heme reduction. For wild type P450 BM3, Kd(red)(laurate)=82.4μm (cf. Kd(ox)=364μm). In addition, an unusual spectral feature in the red region of the absorption spectrum of the reduced P450-4CNPy adduct is observed that can be assigned as a metal-to-ligand charge transfer (MLCT). It was discovered that the energy of this MLCT varies linearly with respect to the P450 heme reduction potential. By studying the energy of this MLCT for a series of BM3 active site mutants with differing reduction potential (Em), the relationship EMLCT=(3.53 x Em)+17,005cm-1 was derived. The use of this ligand thus provides a quick and accurate method for predicting the heme reduction potentials of a series of P450 BM3 mutations using visible spectroscopy, without the requirement for redox potentiometry.
It has also been demonstrated that 4-cyanopyridine can be exploited to predict the substrate-free P450 heme reduction potentials. This feature makes 4-cyanopyridine a fundamentally important mechanistic probe. The energy of the MLCT absorption upon formation of the 4CNPy-ferrous adduct varieslinearly with heme reduction potential.
Effect of the coordination of π-acceptor 4-cyanopyridine ligand on some compounds
Many studies on the 4-cyanopyridine ion iron(III) ion complex types [FeIII(Porph)(4-CNpy)2]+, using the following four meso-porphyrins: (1) the unsubstituted meso-tetraphenylporphyrin (H2TPP), (2) the methyl para-substituted meso-porphyrin(the meso-tertratolylphenylporphyrin H2TTP), (3) the 2,3,4-methyl para-substituted meso-porphyrin (the meso-tetramesitylporphyrin H2TMP) and (4) the OMe para-substituted mesoporphyrin (H2TMPP) have been carried out.[2]
To examine the influence of both the important π-acceptor character of the 4-cyanopyridine ligand and the nature of the para-substituted phenyls of meso-porphyrins on the electronic, electrochemical and structural properties of cobaltous metalloporphyrins, the researchers prepared and fully characterized two coordination compounds: the (4-cyanopyridine)[meso-tetra(para-methoxyphenyl)porphyrinato]cobalt(ii) and the (4-cyanopyridine)[meso-tetra(para-chlorophenyl)porphyrinato]cobalt(ii) with the [CoII(TMPP)(4-CNpy)] and [CoII(TClPP)(4-CNpy)] formulas (complexes 1-2). The solution structures of compounds 1-2 were confirmed by 1H NMR spectroscopy and mass spectrometry methods. They were further characterized by cyclic voltammetry and photoluminescence studies. The X-ray molecular structure data show that the Co-TClPP-4-NCpy derivative (2) exhibits high ruffling deformation compared to that of the Co-TMPP-4-CNpy species (1). Notably, the crystal packing of complex 1 shows the formation of Co⋯Co supramolecular dimers with a distance of 5.663 Å. As an application of our two cobaltous compounds, an investigation involving complexes 1-2 in the degradation of the methylene blue dye in the presence and absence of H2O2 in aqueous solutions was carried out. These promising results show that 1-2 can be used as catalysts in the degradation processes of dyes.
Continuous hydrolysis of 4-cyanopyridine by nitrilases
This study proposed removing the nitrilase by-product isonicotinamide resulting from theaction of F. solani nitrilase on 4-cyanopyridine in a follow-up reaction employing the amidase from Rhodococcus erythropolis A4. The nitrilase and amidase were adsorbed on two Butyl sepharose columns operated inseries.[3]
The operational stabilities of nitrilases from Aspergillus niger K10 and Fusarium solani O1 were examined with 4-cyanopyridine as the substrate in continuous-stirred membrane reactors (CSMRs). The former enzyme was fairly stable at 30℃ with a deactivation constant (kd) and enzyme half-life of 0.014 h-1 and 50 h, respectively, but the latter exhibited an even higher stability characterized by kd = 0.008 h-1 and half-life of 87 h at 40℃. Another advantage of this enzyme was its high chemoselectivity, i.e., selective transformation of nitriles into carboxylic acids, while the amide formed a high ratio of A. niger K10 nitrilase product. High conversion rates (>90%) were maintained for about 52 h using the nitrilase from F. solani O1 immobilized in cross-linked enzyme aggregates (CLEAs). The purity of isonicotinic acid was increased from 98% to >99.9% by using two CSMRs connected in series, the first one containing the F. solani O1 nitrilase and the second the amidase from Rhodococcus erythropolis A4 (both enzymes as CLEAs), the amidase hydrolyzing the by-product isonicotinamide.
Conclusion
Except for 4-cyanopyridine, several cyanopyridine-based drugs have been approved for clinical use in the treatment of various cancers, revealing cyanopyridine's potential as a useful scaffold for developing novel anticancer drugs. Various derivatives with cyano groups attached to pyridine rings have been designed and synthesized, and their pharmacological activities have been studied from various angles, including anticancer,antibacterial (i.e., insecticidal, bacterial, fungal, viral, etc.), anticonvulsant, anti-Alzheimer's disease, and so on.[4]
References
[1] Ost TW, Clark JP, Anderson JL, Yellowlees LJ, Daff S, Chapman SK. 4-cyanopyridine, a versatile spectroscopic probe for cytochrome P450 BM3. J Biol Chem. 2004;279(47):48876-48882. doi:10.1074/jbc.M408601200
[2] Guergueb M, Nasri S, Brahmi J, et al. Effect of the coordination of π-acceptor 4-cyanopyridine ligand on the structural and electronic properties of meso-tetra(para-methoxy) and meso-tetra(para-chlorophenyl) porphyrin cobalt(ii) coordination compounds. Application in the catalytic degradation of methylene blue dye. RSC Adv. 2020;10(12):6900-6918. Published 2020 Feb 14. doi:10.1039/c9ra08504a
[3] Malandra A, Cantarella M, Kaplan O, et al. Continuous hydrolysis of 4-cyanopyridine by nitrilases from Fusarium solani O1 and Aspergillus niger K10. Appl Microbiol Biotechnol. 2009;85(2):277-284. doi:10.1007/s00253-009-2073-x
[4] Wu Y, Wu T, Huang Y. A review: Biological activities of novel cyanopyridine derivatives. Arch Pharm (Weinheim). 2023;356(7):e2300067. doi:10.1002/ardp.202300067
See also
Lastest Price from 4-Cyanopyridine manufacturers

US $10.00/KG2025-04-21
- CAS:
- 100-48-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $68.00/Kg/Bag2025-03-26
- CAS:
- 100-48-1
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 10T