Application of saikosaponin-d in cancer
Background
Autophagy is an important cellular process that controls cells in a normal homeostatic state by recycling nutrients to maintain cellular energy levels for cell survival via the turnover of proteins and damaged organelles. However, persistent activation of autophagy can lead to excessive depletion of cellular organelles and essential proteins, leading to caspase-independent autophagic cell death. As such, inducing cell death through this autophagic mechanism could be an alternative approach to the treatment of cancers. Recently, we have identified a novel autophagic inducer, saikosaponin-d (Ssd), from a medicinal plant that induces autophagy in various types of cancer cells through the formation of autophagosomes as measured by GFP-LC3 puncta formation. By computational virtual docking analysis, biochemical assays and advanced live-cell imaging techniques, Ssd was shown to increase cytosolic calcium level via direct inhibition of sarcoplasmic/endoplasmic reticulum Ca2+ ATPase pump, leading to autophagy induction through the activation of the Ca2+/calmodulin-dependent kinase kinase–AMP-activated protein kinase–mammalian target of rapamycin pathway. In addition, Ssd treatment causes the disruption of calcium homeostasis, which induces endoplasmic reticulum stress as well as the unfolded protein responses pathway. Ssd also proved to be a potent cytotoxic agent in apoptosis-defective or apoptosis-resistant mouse embryonic fibroblast cells, which either lack caspases 3, 7 or 8 or had the Bax-Bak double knockout. These results provide a detailed understanding of the mechanism of action of Ssd, as a novel autophagic inducer, which has the potential of being developed into an anti-cancer agent for targeting apoptosis-resistant cancer cells.
Application of saikosaponin-d in autophagy effect
Autophagy is a highly regulated lysosomal proteolytic degradation process, which can engulf superfluous or damaged organelles and proteins through the formation of double-membraned autophagic vacuoles (autophagosomes). Upon formation of the autolysosomes through fusion of autophagosomes and lysosomes, all engulfed cellular components are degraded by acidic lysosomal hydrolases to synthesize new proteins. Although autophagy has a pivotal role in cellular housekeeping functions, it can be induced by stressors such as nutrients deprivation, oxidative stress, damaged proteins or organelles, pathogenic infection or hypoxia. Furthermore, defects in autophagy are often linked to liver diseases, neurodegenerative diseases, inflammatory diseases, aging, cancers and metabolic syndromes.
Emerging evidence supports the role of autophagy in tumor suppression, as increased levels of autophagy might lead to autophagic cell death (type II programmed cell death) in cancers. Autophagic cell death is accompanied by the formation of autophagosomes/autolysosomes without chromatin condensation. As cancer cells are frequently resistant to drug-mediated apoptosis after long-term chemotherapeutic treatments, the induction of autophagic cell death in apoptosis-defective or apoptosis-resistant tumor cells may provide an alternative therapeutic approach to tumor suppression. Recently, a number of clinically approved or experimental anti-tumor drugs have been shown to induce autophagy-related cell death in some malignant cells. Therefore, the development and use of small-molecule autophagic inducers is an emerging approach for treatment of cancers, especially where they have become apoptosis resistant.
We have previously identified a small-molecule, saikosaponin-d (Ssd), that exhibits anti-inflammatory effect via the suppression of NF-κB, NF-AT and AP-1 signaling. We recently showed that Ssd potentiated the anti-cancer potency of TNF-α through NF-κB inhibition. Ssd is one of the major triterpenoid saponins derived from Bupleurum falcatum L. (Umbelliferae), which is commonly prescribed for inflammatory and infectious diseases, and its active component Ssd was reported to have immuno-modulatory, anti-inflammatory, anti-bacterial, anti-viral and anti-cancer activities.
In this study, we present the first report that Ssd increased autophagic flux in HeLa and MCF-7 cancer cells, causing both apoptosis and autophagic cell death. Mechanistic studies revealed that Ssd-induced autophagy occurred by direct inhibition of sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA), leading to the increase of intracellular calcium ion levels and activating the Ca2+/calmodulin-dependent kinase kinase-β (CaMKKβ)–AMP-activated protein kinase (AMPK)–mammalian target of rapamycin (mTOR) signaling cascade, endoplasmic reticulum (ER) stress and unfolded protein responses (UPR). The activation of these pathways ultimately leads to apoptotic and autophagic cell death in both cancer cells and, more importantly, apoptosis-resistant cancer cells.
Ssd induces autophagy in HeLa and MCF-7 cells
Autophagy is directly involved in reducing the growth of tumor cells, thus chemical autophagy inducers could have the potential of inhibiting tumor cells. By using a panel of cancer cells, including HeLa, MCF-7, HepG2, PC3, H1299 and LLC-1, Ssd were shown to induce green fluorescent protein light-chain 3 (GFP-LC3) puncta formation (a marker of autophagy) in response to Ssd treatment. Among these cell types, HeLa and MCF-7 cells were chosen for more detailed mechanistic study. Results indicated that Ssd could time dependently induce autophagy as shown by the increased number of fluorescence GFP-LC3 puncta observed within the cells. To confirm the Ssd-induced autophagic activity, 5 mM 3-methyladenine (3-MA; an autophagic inhibitor) was added before Ssd treatment. Consistently, the addition of 3-MA decreased the Ssd-mediated autophagy as shown by the decreased percentage of cells with GFP-LC3 puncta (Fig.1 a). In addition, immunocytochemical staining of endogenous LC3-II protein (a component of the autophagosome) also showed an increased level of puncta formation in cells treated with Ssd (Fig.1 b).
Autophagosome accumulation may be caused by either an induction of autophagic flux via the autophagic pathway or an impairment of autophagosomes removal due to the blockage of fusion between autophagosome and lysosome.To differentiate between these two events, LC3-II formation in the presence of lysosomal protease inhibitors was assayed. Whereas Ssd or lysosomal protease inhibitors alone increased the level of LC3-II as expected, Ssd significantly increased the rate of LC3-II expression as well as its GFP-LC3 puncta formation in the presence of the inhibitors, compared with the addition of protease inhibitors alone (Fig.1c and d ). These results therefore suggest that Ssd induces autophagic activity through increased autophagosome formation. p62 protein is another autophagy marker, which is selectively incorporated into autophagosomes and degraded upon autophagy induction. As shown in Fig. e decreased protein levels of p62, at least over the first 4 h of exposure, further suggest that Ssd induces autophagic flux.
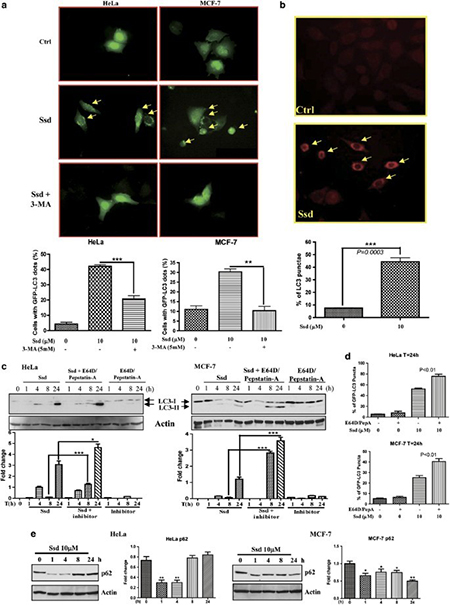
Fig.1 Induction of autophagy by saikosaponin-d (Ssd).
Renference
1 Wong, V., Li, T., Law, B. et al. Saikosaponin-d, a novel SERCA inhibitor, induces autophagic cell death in apoptosis-defective cells. Cell Death Dis 4, e720 (2013).
You may like
Lastest Price from Saikosaponin D manufacturers

US $0.00-0.00/kg2025-04-21
- CAS:
- 20874-52-6
- Min. Order:
- 1kg
- Purity:
- 95%; 98% HPLC
- Supply Ability:
- 1000kg

US $0.00/kg2024-04-12
- CAS:
- 20874-52-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 2000ton