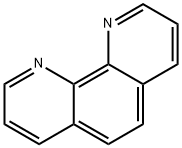
Application of O-Phenanthroline
General description
O-Phenanthroline is an organic compound with the molecular formula C12H8N2. The solid is white crystal and dissolved in water to form a light yellow to yellow solution. When recrystallized with water, it contains a molecule of crystallization water. O-phenanthroline hydrate is white crystal. When recrystallizing with benzene, it does not contain crystal water, and the melting point is 98 ~ 100 ℃ (117 ℃). Boiling point above 360 ℃. Soluble in ethanol, benzene and acetone, insoluble in petroleum ether. It forms complexes with iron, copper, cobalt, nickel and 2,2-bipyridine, forms red complexes with Fe2 +, and oxidizes with potassium permanganate to obtain 2,2-dipyridyl-3,3 ′ - dicarboxylic acid. It is a common redox indicator. It is a bidentate heterocyclic compound ligand, similar to 2,2-bipyridine. It is a common auxiliary ligand in the construction of crystalline materials. It has strong chelation and will form very stable complexes with most metal ions. It can be used as a quantitative colorimetric reagent for copper and iron, and also as an indicator for titrating iron salt with cerium sulfate; It can also be used as a dye for animal fibers.[1]
Application
A chelating agent, forming complexes with most metal ions. A ligand employed in the spectrophotometric determination of metals and photocatalytic reduction of carbon dioxide. As an analytical reagent for determination of metals in chemical and biological systems through complex formation. Phenanthroline solid is white crystal and dissolved in water to form a light yellow to yellow solution. Phenanthroline Fe (II) indicator can be prepared by dissolving 1.485g phenanthroline monohydrate and 0.695g FeSO4 · 7H2O in 100ml water. Indicator for cerium sulfate titration of iron salt. A related ligand is red phenanthroline (BPT), 4,7-diphenyl-1,10-phenanthroline. Phenanthroline can also be used to analyze the content of alkyl lithium compounds. The specific step is to make the sample react with a small amount (about 1mg) of Phenanthroline to be dark, and then titrate with alcohol until the colorless titration end point is reached. In the solution with pH = 2 ~ 9, phenanthroline reacts with ferrous ion (Fe2 +). The selectivity of the reaction is very high, and the orange red complex is very stable, LGK stability = 21.3 (20 ℃).
1.The solution has the maximum absorption peak at 510nm (visible light). Using this color reaction, trace iron can be determined by visible light spectrophotometry. Although the suitable pH range of color reaction is very wide (pH = 2 ~ 9), it is usually measured in HAC NaAc buffer medium with pH = 5. For example:y evaluation of 1,10 - phenanthroline spectrophotometric method for the determination of iron in sodium - hydroxide and analyzing its source of uncertainty By establishing mathematical model.The result shows that the content of iron in sodium-hydroxide is( 1.203±0.0287)×10-3%,the extended-uncertainty is 2.87×10-5%,k=2.The main source of uncertainty is from fitting of standard curve and preparation of standard solution.
2.Electron mobility is an important parameter of electron transport materials, which has an important impact on the power efficiency of electroluminescent devices. 1,10-phenanthroline unit has a rigid, electron deficient planar structure, which is easy to obtain high electron mobility when applied to electron transport materials, such as common electron transport / hole barrier materials bphen, BCP, etc., and the electron mobility reaches 10−4 cm2 V-1 s-1. However, the electron transport materials based on 1,10-phenanthroline are also easy to crystallize, which has an impact on the stability of the device. For example, the glass transition temperature of bphen is 66 ℃, while BCP is a crystalline material. Therefore, it is necessary to improve the glass transition temperature and electron mobility of organic electron transport materials at the same time. In addition, the 1,10-phenanthroline unit has a wide energy gap and deep HOMO energy level, which is conducive to improve the hole blocking performance of the material.[2]
3.Carbohydrates are essential moieties of many bioactive molecules in nature. However, efforts to elucidate their modes of action are often impeded by limitations in synthetic access to well-defined oligosaccharides. Most of the current methods rely on the design of specialized coupling partners to control selectivity during formation of glycosidic bonds. Herein, we report a commercially available phenanthroline to catalyze stereoretentive glycosylation with glycosyl bromides. The method provides efficient access to α−1,2-cis glycosides. This protocol has been performed for the large-scale synthesis of an octasaccharide adjuvant. Density functional theory calculations, together with kinetic studies, suggest that the reaction proceeds via double SN2 mechanism. Catalytic Glycosylations: The commercially available phenanthroline serves as the efficient catalyst to promote the stereoselective formation of -1,2-cis glycosides synthesis. This newly developed catalyst system is not confined to the nature of the protecting groups bound on carbohydrate coupling partners to effect the selectivity.[3] Pyridine-mediated reaction proceeds with marginal bias for the α-selectivity. Herein, we report the discovery of a commercially available phenanthroline to stereoselectively catalyze formation of α−1,2-cis glycosides (Scheme 1C). Phenanthroline is a rigid and planar structure with two fused pyridine rings whose nitrogen atoms are positioned to act cooperatively. We postulated that the first nitrogen atom serves as a catalytic nucleophile to react with an electrophile to generate a covalent β-glycosyl phenanthrolium ion preferentially as phenanthroline is more sterically demanding than pyridine.
Figure 1 Mechanistic studies
Storage and Safety
White powder can be toxic if swallowed., It is highly toxic to aquatic organisms and has long-term and sustained effects. Consult a doctor., Show this safety technical instruction to the doctor on site. If inhaled, remove the patient to fresh air., If breathing stops, perform artificial respiration., Consult a doctor. Rinse with soap and plenty of water., Send the patient to the hospital immediately., Consult a doctor. Wear gloves before use. Please remove the gloves with appropriate methods (do not touch the outer surface of the gloves) and avoid any skin parts from contacting the product After use, please handle the contaminated gloves carefully according to relevant laws and regulations and effective laboratory rules and procedures Please wash and dry your hands. The protective gloves selected must meet the specifications given in regulation (EU) 2016 / 425 and en 374 standard derived from it. If safety can be ensured, measures can be taken to prevent further leakage or overflow. Do not allow the product to enter the sewer. Avoid discharge into the surrounding environment.
Reference
1.Evaluation of uncertainty in the determination of iron content in industrial sodium hydroxide by 1,10-phenanthroline Spectrophotometry.
2.Wei Xinfeng, Containing 1,10-phenanthroline units, there are Application in organic electroluminescent devices.
3.Yu F., Li J. & DeMent P. M. et al., "Phenanthroline-Catalyzed Stereoretentive Glycosylations," Angew Chem Int Ed Engl, Vol.58, No.21(2019), pp.6957-6961.
You may like
Related articles And Qustion
Lastest Price from o-Phenanthroline manufacturers

US $10.00/KG2025-04-21
- CAS:
- 66-71-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/KG2025-04-15
- CAS:
- 66-71-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg