1,10-phenanthroline: Chemical properties, applications, and future prospects
Abstract
In this paper, the chemical properties, applications, and future development prospects of 1,10-phenanthroline were reviewed. First, the basic chemical properties, structural characteristics, and molecular structure are introduced. Secondly, its applications in metal ion detection, drug development, materials science, and photochemistry are described in detail. Finally, its future development is prospected.
Introduction
1,10-phenanthroline, as an important organic compound, has shown wide application potential in the fields of chemistry, biology, and materials science. Its unique molecular structure and chemical properties make it a key molecule in many chemical reactions and biological processes. In this paper, its chemical properties, application fields, and future development prospects will be reviewed.

Chemical characteristics
1. Chemical Formula and structure:
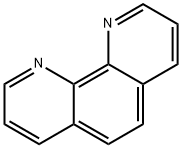
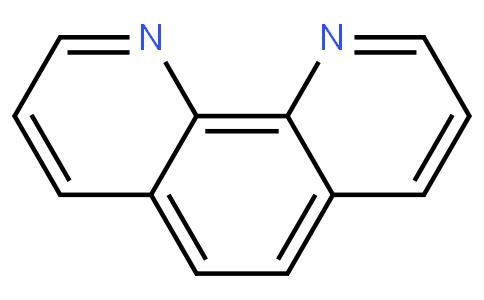
The chemical formula of 1,10-phenanthroline is C₁₂H₈N₂ with a molecular weight of 180.205. Its molecular structure consists of two benzene rings connected by a nitrogen atom to form a plane molecule with a large conjugated system. This structure gives it unique stability and optical properties.1
2. Physical properties:
white to light yellow crystalline powder at room temperature, melting point is 117℃ (anhydrous matter). It is soluble in water and a variety of organic solvents, such as ethanol, acetone and so on.2
3. Chemical properties:
It has excellent coordination ability and can form stable complexes with a variety of metal ions. These complexes usually have a specific color and absorption spectrum, which provides convenience for the detection and quantitative analysis of metal ions.3
Application fields
1. Metal ion detection
1,10-phenanthroline has been widely used in metal ion detection. Due to the specific color and absorption spectrum of the complexes formed with metal ions, the content of metal ions can be quickly and accurately detected by colorimetry or spectrophotometry. For example, a complex formed with ferrous ions (Fe2+) has a maximum absorption peak at 510nm and can be used for the detection of trace amounts of iron. In addition, it can also be used for the detection of metal ions such as copper, nickel, and cobalt.3
2. Drug research and development
In the field of drug research and development, it can be used as the skeleton or ligand of drug molecules to bind with different drug-active groups to form drug molecules with specific biological activity. Some compounds containing 1,10-phenanthroline structure have been shown to have anti-cancer, antibacterial, and other biological activities. In addition, it can also be used as a carrier of a drug delivery system to effectively transport drug molecules to target tissues or cells to achieve precise treatment of drugs.4
3. Materials science
In the field of materials science, the application of 1,10-phenanthroline is mainly reflected in the preparation of metal-organic frameworks (MOFs) and photosensitive materials. Due to their unique coordination capabilities and stability, they can be used to build MOF materials with specific structures and functions. These materials show good application prospects in the fields of gas adsorption, separation, and catalysis. In addition, it can also be used as a ligand of photosensitive materials for the preparation of materials with excellent photoelectric properties.
4. Photochemistry
In the field of photochemistry, 1,10-phenanthroline, as an excellent photosensitizer, can be used in the study and application of photochemical reactions. Its unique molecular structure and optical properties have potential applications in photocatalysis and photoluminescence.5
prospects
With the continuous development of science and technology and the continuous expansion of application fields, the application prospect of 1,10-phenanthroline will be broader. In the future, people will further explore new application fields and new functions, and develop new synthesis methods and modification technologies to improve their performance and application effects. At the same time, people will also pay attention to its environmental friendliness and sustainable development issues, to achieve its wide application in green chemistry and circular economy.
References
[1].Sammes, P. G.; Yahioglu, G., 1, 10-Phenanthroline: a versatile ligand. Chemical Society Reviews 1994,23(5), 327-334.
[2].Sigman, D. S., Nuclease activity of 1, 10-phenanthroline-copper ion. Accounts of Chemical Research 1986, 19(6),180-186.
[3].Corey, E.; Borror, A.; Foglia, T., Transformations in the 1, 10-phenanthroline series. The Journal of Organic Chemistry 1965,30(1), 288-290.
[4].Alreja, P.; Kaur, N., Recent advances in 1, 10-phenanthroline ligands for chemosensing of cations and anions. RSC Advances 2016,6(28), 23169-23217.
[5].Brandt, W. W.; Dwyer, F. P.; Gyarfas, E. D., Chelate complexes of 1, 10-phenanthroline and related compounds. Chemical Reviews 1954,54(6), 959-1017.
Related articles And Qustion
Lastest Price from o-Phenanthroline manufacturers

US $10.00/KG2025-04-21
- CAS:
- 66-71-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00-0.00/KG2025-04-15
- CAS:
- 66-71-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg