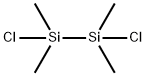
Application of 1,2-dichlorotetramethyldisilane
1,2-dichlorotetramethyldisilane (1,2-Dichloro, 1,1,2,2-Tetramethyldisilane; 1,2-dichloro-1,1,2,2-tetramethyl-disilan; 1,2-Dichloro-1,1,2,2-tetramethyldisilane; 1,2-dichloro-1,1,2,2-tetramethyl-disilane; 1,2-DICHLOROTETRAMETHYLDISILANE; DICHLOROTETRAMETHYLDISILANE; Disilane,1,2-dichloro-1,1,2,2-tetramethyl; 1,2-DICHLOROTETRAMETHYLDISILANE, 95+%) is a kind of widely used organic silicon intermediates and usually be used as organic silicon blocking agent in the synthesis,such as,Si-Si Compounds; Dichlorosilanes (for Polysilanes); Electroluminescence; Functional Materials; Monochlorosilanes; Reagent for High-Performance Polymer Research; Si (Classes of Silicon Compounds); Si-Cl Compounds; Silicon Compounds (for Synthesis); Synthetic Organic Chemistry; Precursors by Metal; Vapor Deposition Precursors[1].

Generally, This product, 1,2-dichlorotetramethyldisilane (1), was synthesized according to from the ‘disilane fraction’ of the direct chloromethylsilane synthesis, varied only by using Bu3N as the catalyst for the final Si - Si cleavage of the higher chlorinated disilanes by HCl[2]. In addition, the effect of the temperature on the composition and ratio of hydrolysis products of 1,2 dichlorotetramethyldisilane was explored comprehensively. The lowtemperature hydrolysis of dichlorodisilane 1 gives cyclic and linear permethyl (oxadisilanes) by the analysis of GC-MS data. the Raman vibrational spectra of 1,2-dichlorotetramethyldisilane recorded at various temperatures prove that two rotamers, gauche and anti, are present in the liquid state due to restricted rotation of the SiMe2Cl groups. The solid is composed of the anti rotamer exclusively[3].
Besides, It is used as an intermediate for preparing other organical compounds. It is also used in aqueous polymer preparation as well as a laboratory reagent. 1,2-Dihydroxy-tetramethyldisilane (2) has been synthesized by hydrolysis of 1,2-dichloro-tetramethyldisilane (1) and characterized by IR, NMR, MS and X-ray structure analysis. Compound 2 exists in crystal form in two different conformations forming a characteristic two-dimensional network by H-bonding. The title compound is water soluble and has a high condensation tendency[4]. And 1,2-Dihydroxy-tetramethyldisilane (2) could be prepared in a very simple manner by hydrolysis of 1,2-dichloro-tetramethyldisilane (1) in a homogeneous ether/acetone mixture with triethyl amine as the HCl acceptor[4].
Munirathina[5] et al. synthesized Two new diaminodisilanes, 1,2-bis(diethylamino)tetramethyldisilane and 1,2 - bis(anilino)tetramethyldisilane from 1,2-dichlorotetramethyldisilane and the respective amines. These disilane-containing monomers were reacted with bisphenols containing amide and imide groups, giving disilane-containing polyamide and polyimides which had rather low inherent viscosity around 0.1 dlg-1. The polymers were characterized by UV, IR, and elemental analysis. All the polymers were soluble in N,N-dimethylacetamide, dimethyl sulfoxide, Nmethyl-2-pyrrolidone, and some of them were also soluble in tetrahydrofuran. The polymers had glass transition temperatures between 105-120°C and were thermally stable up to 300 0C in both air and nitrogen atmospheres. A decrease in the moleclar weight was observed upon exposure to UV light indicating the photosensitivity of the disilane-containing polymers.
References
[1] https://www.chemicalbook.com/ProductChemicalPropertiesCB4280343_EN.htm
[2] Matsumoto H, Motegi T, Hasagawa M, et al. A convenient and large scale synthesis of 1, 1, 2-trimethyl-1, 2, 2-trichlorodisilane and 1, 1, 2, 2-tetramethyl-1, 2-dichlorodisilane[J]. Journal of Organometallic Chemistry, 1977, 142(2): 149-153.
[3] Chernyavskii A I, Pleshkova A P. Effect of the temperature on the composition and ratio of hydrolysis products of 1, 2-dichlorotetramethyldisilane[J]. Russian chemical bulletin, 2006, 55(4): 748-750.
[4] Prasse M, Reinke H, Wendler C, et al. Synthesis, structure and properties of 1, 2-dihydroxy-tetramethyldisilane[J]. Journal of organometallic chemistry, 1999, 577(2): 342-345.
[5] Padmanaban M, Kakimoto M, Imai Y. Preparation and properties of new disilane-containing polyamide and polyimides from diaminodisilanes and bisphenol compounds[J]. Polymer journal, 1990, 22(7): 587.