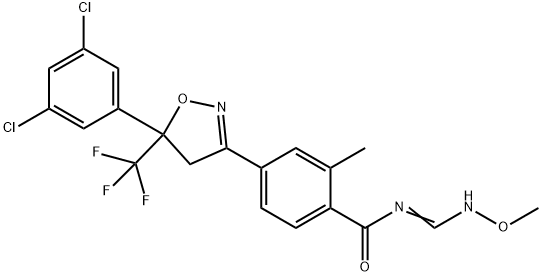
An isoxazoline insecticide- Fluxametamide
Description
Fluxametamide was announced by Nissan Chemical Industries as a new insecticide in 2015. It is a novel neuroactive isoxazoline compound that binds to a unique site on the γ-aminobutyric acid (GABA) receptor and has exceptional insecticidal activity on a range of lepidopteran caterpillars[1]. It is, just as the closely related animal health products fluralaner and afoxolaner, acting as a non-competitive antagonist of the GABA-gated chloride channel in insects at a novel allosteric site distinct from that to which fiproles and cyclodienes bind. Fluxametamide and the related parasiticides fluralaner and afoxolaner are reportedly much less active on mammalian GABA receptors. A recent study revealed that the (S)-enantiomer of fluxametamide shows higher insecticidal potency and lower risk to honeybees than the commercialized racemate.;
Synthesis method
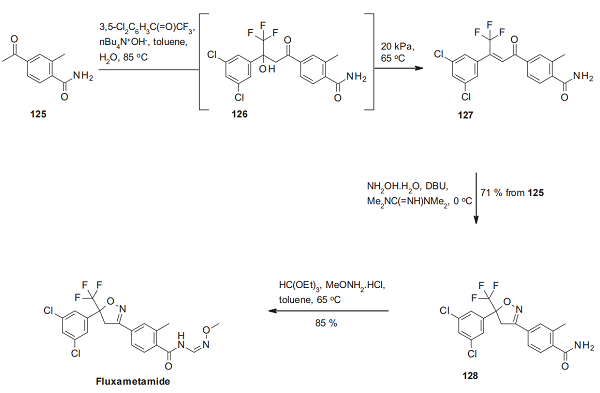
The synthesis of fluxametamide starts with an aldol condensation of the methyl ketone 125 with 3 ′,5 ′ -dichloro-2,2,2-tri- fluoroacetophenone under phase-transfer conditions. The resulting 126 is dehydrated in situ by azeotropic water removal to the chalcone derivative 127, which is cyclized with hydroxylamine hydrate to the isoxazoline derivative 128. Subsequent condensation of the primary amide in 128 with triethyl orthoformate and O-methylhydroxylamine delivers fluxametamide as an E/Z mixture, which is transformed into the desired (Z)-isomer by dynamic re-crystallization[2].
Toxicity
Alveolar macrophage accumulation, vacuolated epithelial cells in the small intestine, and hepatocellular vacuolation are observed in various toxicity studies of fluxametamide. Fluxametamide showed neither neurotoxicity, reproductive toxicity, teratogenicity, nor genotoxicity. Increased incidences of thyroid follicular cell adenoma in male rats and of hepatocellular adenoma in male mice were observed in carcinogenicity studies. However, a genotoxic mechanism was unlikely to be involved in the tumor increases[3]. It was thus reasonably considered to establish a threshold dose in the assessment. The lowest no-observed-adverse-effect level (NOAEL) obtained in all studies was 0.85 mg/kg bw per day in rats' two-year combined chronic toxicity/carcinogenicity study. FSCJ specified an acceptable daily intake (ADI) of 0.0085 mg/kg bw per day, applying a safety factor of 100 to the NOAEL. FSCJ considered it unnecessary to specify an acute reference dose (ARfD) since fluxametamide is unlikely to exert toxic effects after a single oral dose administration.
References
[1] Debashis Roy . “Resistance risk assessment in diamondback moth, Plutella xylostella (L.) to fluxametamide.” Crop Protection 163 (2023): Article 106101.
[2] Stephane Jeanmart . “Synthetic approaches to the 2015–2018 new agrochemicals.” Bioorganic & Medicinal Chemistry 39 (2021): Article 116162.
[3] “Fluxametamide (Pesticides).” Food safety (Tokyo, Japan) 8 1 (2020): 10–11.
You may like
See also
US $1.00-0.50/kg2025-04-21
- CAS:
- 928783-29-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 200kg