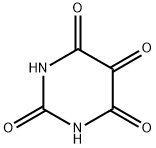
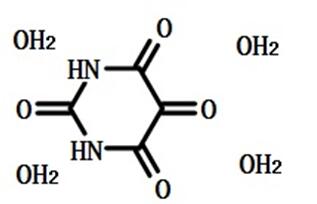
Alloxan: Drugs Used Experimentally for the Induction of Diabetes
Alloxan is white orthorhombic crystal and turns pink when exposed to air or heated to 230 ° C or higher, and UV λ is max 243 nm at pH 6 in water. It usually contains a crystal water, which becomes an anhydride when heated to 170 ° C. Its melting point is 256 ° C (decomposition) It is miscible with water. Hot aqueous solutions are yellow and become colorless on cooling. Soluble in ethanol, acetone, glacial acetic acid, slightly soluble in chloroform, toluene, petroleum ether, acetic anhydride, insoluble in ether.
Aqueous solutions (acidic), after being in contact with the human skin for some time, give it a red color and a disagreeable odor. It can form an adduct with NaHSO3; react with HNO2 to form oxalyl urea; and also be reduced to obtain diurea; auric acid can be first produced through its alkaline hydrolysis to obtain acetone diacid and urea. It turns dark blue when reacting with iron salt. It is found in dysentery mucus.
Alloxan can be produced by the reaction of diurea and fuming nitric acid, or by oxidation of uric acid (2,6,8-trihydroxyindole) by nitric acid, or by condensation of diethyl adipic acid and urea.
In general, it is used in biochemical research and organic synthesis, also used as a color developer for iron, cobalt, nickel, manganese and other ions. In addition, it’s used for anti-tumor in pharmaceuticals.
Drugs Used Experimentally for the Induction of Diabetes
Alloxan [2,4,5,6-tetraoxypyrimidine; 5,6-dioxyuracil] was first noted to have diabetogenic effects in 1943 when central islet necrosis was found in alloxan-treated rabbits (Dunn et al., 1943). From then on, it has been used to model Type 1 diabetes in animal experimentation.
Alloxan and streptozotocin are the two agents most widely used to induce experimental diabetes. Alloxan-induced destruction of the pancreatic islet beta cell produces permanent diabetes mellitus in a wide range of species and, in the appropriate dose, it is selective to islet beta cell, producing minimal effects on other structures (Cooperstein and Watkins, 1981).
Alloxan is selectively toxic to insulin-producing pancreatic beta cells because it preferentially accumulates in beta cells through uptake via the GLUT2 glucose transporter. Alloxan, in the presence of intracellular thiols, generates reactive oxygen species (ROS) in a cyclic reaction with its reduction product, dialuric acid. The beta cell toxic action of alloxan is initiated by free radicals formed in this redox reaction. Studies suggests that alloxan does not cause diabetes in humans. Others found a significant difference in alloxan plasma levels in children with and without diabetes Type 1.
Reference
National Institute for Occupational Safety and Health (NIOSH), Manual of Analytical Methods, 4th ed., Method 8005, U. S. Department of Health and Human Services, Washington, DC, 1994.
National Institute for Occupational Safety and Health (NIOSH), Manual of Analytical Methods, 4th ed., Method 8310, U.S. Department of Health and Human Services, Washington, DC, 1994.
F. Sunderman, Determination of nickel in water body fluids, tissues and excreta. In J. K. O’Neill, P. Schuller, and L. Fishbein, eds., Environmental Carcinogens: Selected Methods of Analysis, IARC Sci. Publ. No. 71, IARC, Lyon, France, 1986, pp. 319–334.
J. R. Andersen, B. Gammelgaard, and S. Reimert, Direct determination of nickel in human plasma by Zeeman-corrected atomic absorption spectrometry. Analyst (London) 111, 721–722 (1986).