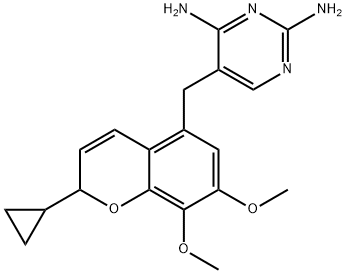
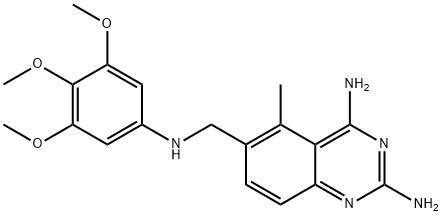
Adverse effects of Iclaprim
Iclaprim (previously known as AR-100 and Ro 48-2622) is a new member of the diaminopyrimidine class of antimicrobials, to which trimethoprim also belongs. As with trimethoprim, iclaprim is an inhibitor of bacterial dihydrofolate reductase (DHFR), an enzyme in the folate synthesis pathway. The two potential advantages of iclaprim compared with trimethoprim are an increased affinity for bacterial DHFR, particularly in Gram-positive bacteria, and activity against trimethoprim-resistant organisms. Iclaprim is therefore a potentially interesting new agent for the treatment of multiresistant Gram-positive infections.
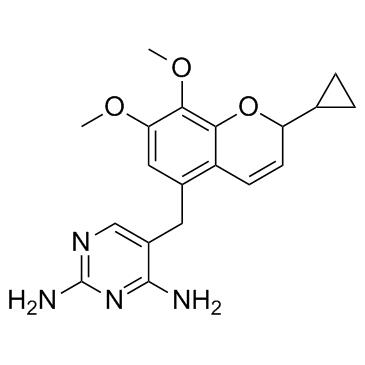
Pharmacological activity
Iclaprim is active against Gram-positive bacteria including Staphylococcus aureus (including methicillin-resistant, and vancomycinintermediate and resistant strains), streptococci and enterococci. It also has limited in vitro activity against Gram-negative pathogens including Enterobacteriaceae and respiratory pathogens such as Haemophilus influenzae, Moraxella catarrhalis, Legionella pneumophila and Chlamydophila pneumoniae.
Mechanism of action
Iclaprim, like trimethoprim, is a diaminopyrimidine. Similar to a number of new diaminopyrimidines, iclaprim was designed using molecular modeling to enhance avidity with bacterial DHFR and to overcome trimethoprim resistance. Both antibiotics are competitive inhibitors of bacterial DHFR, the final of six enzymes in the pathway for biosynthesis of tetrahydrofolic acid (THF).
THF is required as a coenzyme for a number of key cellular reactions, including the synthesis of amino acids and nucleic acids. Thus, diaminopyrimidines block the synthesis of DNA, RNA, and proteins. Isothermal titration calorimetry and co-crystallization techniques demonstrate that iclaprim has a larger binding surface area and increased hydrophobic interactions with DHFR from both trimethoprim-sensitive and -resistant S. aureus than trimethoprim, reflected in lower IC50 and Ki values. The use of diaminopyrimidines as antibacterial agents relies on the diversity between eukaryotic and prokaryotic DHFR and the inability of most bacteria to utilize preformed folate, thus depending on the de novo biosynthesis pathway. The IC50 of iclaprim for human and S. aureus DHFR is W300 and 0.0034 mM, respectively – thus iclaprim has a major differential effect.
Adverse effects
Adverse effects either ''possibly'' or ''probably'' related to iclaprim
during the ASSIST-2 clinical trial. This phase III
study enrolled hospitalized adults with cSSTI and excluded patients
with severe renal or hepatic impairment or morbid obesity (BMI
W40 kg/m2). The most common side-effects were gastrointestinal,
abnormalities in liver enzymes, and neurological.
The effect of iclaprim on QTc interval was also assessed during the
ASSIST trials. Patients taking class IA or class III antiarrhythmics and patients with congenital or sporadic QTc
prolongation were excluded from the study. Compared with the initial
pretreatment electrocardiogram (ECG), the mean maximal QTc
interval in patients receiving iclaprim increased by 7.1 ms (s.d. 13.5)
and 4.0 ms (s.d. 17.0) on ECGs taken on day 1 and day 4 post dose,
respectively. There were no cardiac events attributable to iclaprim.