Acrylonitrile - a Brief Introduction
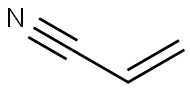
Acrylonitrile, also known as AN or vinyl cyanide, is a man-made VOC. It is a pungent smelling, colorless flammable liquid with the chemical formula CH2CHCN. Its vapors are highly flammable and can explode when exposed to an open flame. Five companies produce AN in the USA, and they produced a total of 2.5 billion pounds in 1993. AN is most commonly used to make acrylic and modacrylic fibers, but can also be used to produce high-impact plastics, packaging plastics, adiponitrile (a chemical involved in the production of nylon), dyes, drugs, and pesticides.
Physical Properties
Acrylonitrile is a clear, colorless to pale-yellow liquid with molecular formula C3H3N and molecular weight of 53.06. The yellowing color is upon exposure to light and indicate photo-alteration to saturate derivate. It is practically odorless, or with a very slight odor that may be describe as sweet, irritating, unpleasant, onion or garlic-like or pungent.
Uses
Acrylonitrile , also known as AN or vinyl cyanide, is a man-made VOC. It is a pungent smelling, colorless flammable liquid with the chemical formula CH2CHCN. Its vapors are highly flammable and can explode when exposed to an open flame. AN is most commonly used to make acrylic and modacrylic fibers, but can also be used to produce high-impact plastics, packaging plastics, adiponitrile (a chemical involved in the production of nylon), dyes, drugs, and pesticides.

Acrylonitrile is primarily used in the manufacture of acrylic and modacrylic fibers. It is also used as a raw material in the manufacture of plastics (acrylonitrile-butadiene-styrene and styrene-acrylonitrile resins), adiponitrile, acrylamide, and nitrile rubbers and barrier resins. A mixture of acrylonitrile and carbon tetrachloride was used as a pesticide in the past; however, all pesticide uses have stopped. Acrylonitrile is a commercially important industrial chemical that has been used extensively since 1940s with the rapid expansion of the petrochemical industry.
Synthesis
Acrylonitrile is produced by catalytic ammoxidation of propylene, also known as the SOHIO process. In 2002, world production capacity was estimated at 5 million tonnes per year,[5][12] rising to about 6 million tonnes by 2017.Acetonitrile and hydrogen cyanide are significant byproducts that are recovered for sale.[5] In fact, the 2008–2009 acetonitrile shortage was caused by a decrease in demand for acrylonitrile.[14]
2 CH3−CH=CH2 + 2 NH3 + 3 O2 → 2 CH2=CH–C≡N + 6 H2O
In the SOHIO process, propylene, ammonia, and air (oxidizer) are passed through a fluidized bed reactor containing the catalyst at 400–510 °C and 50–200 kPag. The reactants pass through the reactor only once, before being quenched in aqueous sulfuric acid. Excess propylene, carbon monoxide, carbon dioxide, and dinitrogen that do not dissolve are vented directly to the atmosphere, or are incinerated. The aqueous solution consists of acrylonitrile, acetonitrile, hydrocyanic acid, and ammonium sulfate (from excess ammonia). A recovery column removes bulk water, and acrylonitrile and acetonitrile are separated by distillation. Historically, one of the first successful catalysts was bismuth phosphomolybdate (Bi9PMo12O52) supported on silica as a heterogeneous catalyst. Further improvements have since been made.
Health Hazard
The EPA has declared AN an air toxic. Health effects on humans and animals depend on the concentration and time of exposure. The health of the person or animal exposed, as well as the conditions of the environment, can also influence the effects of exposure. The symptoms of acute toxicity for AN resemble those of cyanide, which can adversely affect the nervous system, the blood, the kidneys, and the liver. There have been many instances of child mortality due to exposure to AN vapors, whereas adults experienced only mild symptoms when exposed to the same environment.
Contact allergens
Acrylonitrile is a raw material used extensively in industry, mainly for acrylic and modacrylic fibers, acrylonitrile-butadiene-styrene and styrene-acrylonitrile resins, adiponitrile used in nylon’s synthesis, for nitrile rubber, and plastics. It is also used as an insecticide. This very toxic and irritant substance is also a sensitizer and caused both irritant and allergic contact dermatitis in a production manufacturer.
Potential Exposure
Acrylonitrile is used in the manufacture of synthetic fibers, polymers, acrylostyrene plastics, acrylonitrile butadiene styrene plastics, nitrile rubbers, chemicals, and adhesives. It is also used as a pesticide. In the past, this chemical was used as a room fumigant and pediculicide (an agent used to destroy lice).
Carcinogenicity
Acrylonitrile is reasonably anticipated to be a human carcinogenbased on sufficient evidence of carcinogenicity from studies in experimental animals.
You may like
Related articles And Qustion
Lastest Price from Acrylonitrile manufacturers

US $1.00/KG2025-04-21
- CAS:
- 107-13-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $9.00/kg2024-11-07
- CAS:
- 107-13-1
- Min. Order:
- 1kg
- Purity:
- 99.5%
- Supply Ability:
- 200000