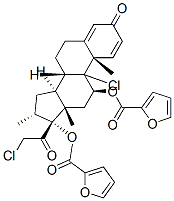
A widely used anti-inflammatory agent-Mometasone furoate
Description
Mometasone furoate is a potent synthetic steroid with a very high receptor affinity and a low bioavailability and has shown to be superior compared to other inhaled corticosteroids. It is always used as a topical steroidal antiinflammatory useful in the treatment of corticosteroid-responsive dermatoses. Mometasone furoate, not mometasone, is used in medical products.
Uses
As a corticosteroid drug, Mometasone furoate is used in various pharmaceutical dosage forms, mainly nasal spray, nasal implant, inhalation powder, topical cream, lotion, ointment, etc. for wide therapeutic applications. It was patented in 1981 and came into medical use in 1987. Mometasone is in a class of medications called corticosteroids. It works by activating natural substances in the skin to reduce swelling, redness, and itching[1].
Mometasone furoate is used in the treatment of inflammatory skin disorders (eczema and psoriasis), allergic rhinitis (hay fever), asthma for patients unresponsive to less potent corticosteroids, and penile phimosis[2]. In terms of steroid strength, it is more potent than hydrocortisone and less potent than dexamethasone. The poor bioavailability of Mometasone furoate in vivo is also a concern. To solve this problem, Zimath loaded MF into zein NPs aiming to evaluate possible advantages that could result from oral delivery and extend the range of MF applications such as inflammatory gut diseases[3].
Side effects
As a widely used anti-inflammatory, its serious side effects are rare, but if Mometasone furoate is used for a long time and in large quantities, it may cause side effects. The nasal spray form of mometasone may cause headaches, Viral upper respiratory infections, sore throat, nose bleeds, cough, and muscle and joint pain. The inhaled form of mometasone for asthma may cause headache, stuffy or runny nose, dry throat, swelling of nose, throat, and sinuses, flu-like symptoms, and painful menstrual periods. The topical (skin cream) version may cause: burning and itching at the application site, acne, changes in skin color, dryness at the application site, and skin sores suggests no clinically relevant pharmacokinetic interactions between indacaterol (IND), glycopyrronium (GLY) and mometasone furoate (MF)[4].
References
[1] Meteran H, et al. Mometasone furoate nasal spray for the treatment of asthma. Expert Opinion on Investigational Drugs, 2016;25.
[2] Taléns-Visconti R, et al. New Vehiculation Systems of Mometasone Furoate for the Treatment of Inflammatory Skin Diseases. Pharmaceutics, 2022; 14: 2558.
[3] Zimath P, et al. Zein nanoparticles as oral carrier for mometasone furoate delivery. Drug Delivery and Translational Research, 2023.
[4] Far J, et al. Developing Biodegradable Nanoparticles Loaded with Mometasone Furoate for Potential Nasal Drug Delivery. ACS Omega, 2020; 5: 7432–7439.
Related articles And Qustion
See also
Lastest Price from Mometasone furoate manufacturers

US $0.00/Kg/Bag2025-04-21
- CAS:
- 83919-23-7
- Min. Order:
- 10g
- Purity:
- 97%-102%; USP
- Supply Ability:
- 10kg

US $0.00-0.00/kg2025-04-21
- CAS:
- 83919-23-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10

![25895-60-7 Sodium cyanoborohydrideNa[BH3(CN)]NaBH4reducing agentCN](/NewsImg/2023-09-19/6383073684614857163220416.gif)