A transition metal boride: zirconium diboride
Introduction
Among borides, zirconium diboride (ZrB2) deserves special attention. ZrB2, with a melting temperature of 3245 ℃, is a member of a family of materials known as ultra-high temperature ceramics (UHTCs). The presence of Zr-Zr metallic bonds results in high electrical and thermal conductivities, whereas B-B covalent bonds improve hardness and chemical inertness. In addition, ZrB2 is resistive against plasma and sparks[1].
Uses
Zirconium diboride (ZrB2) is a transition metal boride. It is characterized by high strength retention at elevated temperatures, high hardness, great thermal shock resistance, erosion, corrosion resistance, high thermal and electrical conductivities, and chemical inertness against molten metals and slag. This makes it suitable for the extreme chemical and thermal environments associated with, for example, hypersonic flight, atmospheric re-entry, and rocket propulsion.
UHTCs possess a unique combination of properties that make them suitable for various applications such as armor materials, cutting tools, specific components for scramjet propulsion, refractory crucibles, wear-resistant parts, rocket nozzles, nose caps, and wings leading edge of hypersonic vehicles.
Structure
According to the Zr–B phase diagram, there are three phases, namely, ZrB, ZrB2, and ZrB12, which have been reported and widely studied for this system. Theoretical investigations show that ZrB can create different crystallographic structures[2]. The basic phase is NaCl-type face-centered cubic ZrB (Fm-3m-space group, no. 225) with lattice constant a = 4.900 Å. Furthermore, ZrB can also crystallize in a FeB-type structure with a primitive orthorhombic (Pnma) crystal structure, a CrB-type orthorhombic structure with a Cmcm-space group, and a hexagonal Pmmm. A literature review shows that ZrB12 is only stable in one structure type. The cubic LuB12 structure (Fm3m-space group, no. 225) with lattice constant a = 7.4085 Å was studied theoretically and experimentally.
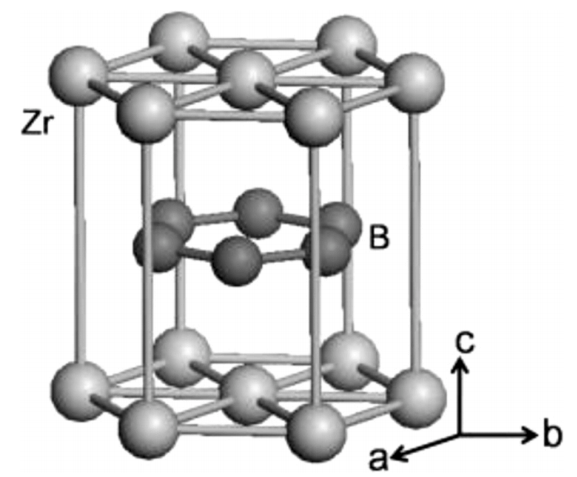
ZrB2 possesses a hexagonal crystal structure featuring a combination of metallic (Zr-Zr) bonds, strong ionic/covalent bonds (Zr-B), and strong covalent bonds (B-B), resulting in a high melting point of around 3250 °C. ZrB2 displays a primitive hexagonal crystal structure with space group P6/mmm. Alternate stacking sequence of zirconium (Zr) planes with graphite-like boron (B) network along c direction results in anisotropy. More precisely, closed-packed zirconium (Zr) layers are alternating with 2D graphene-type sheets made of 12 boron (B) atoms surrounding zirconium atoms. Each zirconium (Zr) atom is surrounded by six equidistant zirconium (Zr) neighbors and 12 equidistant boron (B) atoms. In comparison, each boron (B) atom has six equidistant zirconium (Zr) atoms and three equidistant B atoms.
References
[1] Johnson Frank Guria, Vimal Kumar, Ankit Bansal . “Effect of additives on the thermal conductivity of zirconium diboride based composites – A review.” Journal of The European Ceramic Society 41 1 (2021): Pages 1-23.
[2] Marcin Maździarz, Tomasz Mościcki. “New Zirconium Diboride Polymorphs-First-Principles Calculations.” Materials 13 13 (2020).
You may like
See also
Lastest Price from Zirconium Boride manufacturers

US $0.01-1.00/KG2024-05-13
- CAS:
- 12045-64-6
- Min. Order:
- 25KG
- Purity:
- 99%
- Supply Ability:
- 500 tons

US $100.00/kg2023-09-07
- CAS:
- 12045-64-6
- Min. Order:
- 25kg
- Purity:
- 99%
- Supply Ability:
- 500t/month