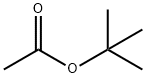
A Research on the toxicity of tert-Butyl acetate
Description
Tert-butyl acetate is a readily available low-cost solvent produced industrially from acetic acid and isobutylene. Its good safety profile makes it suitable for widespread use in catalytic direct amidation reactions[1].
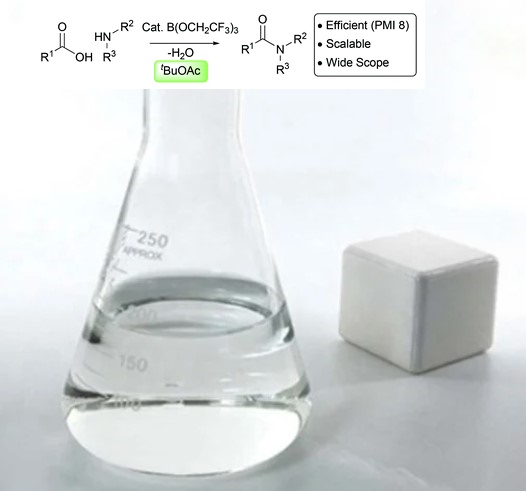
Uses
Tert-Butyl acetate (TBAc) is an organic solvent with potential uses in architectural coatings and as a degreaser. Tert-butyl acetate could be used as the tert-butyl cation source in the Ritter reaction. In addition, tert-butyl acetate is a very effective solvent for B(OCH2CF3) 3-catalyzed direct amidation reactions. The reactions can be run at 1 M concentration, and the amides can typically be purified using a simple resin-based filtration process with low solvent requirements[2-3].
Toxicity
The US Environmental Protection Agency (US EPA) has published a rule proposing the exclusion of TBAc from the definition of Volatile Organic Chemical (VOC) compounds for purposes of Federal regulations related to attaining the national ambient air quality standards (NAAQS) for ozone under title I of the Clean Air Act (Act). This proposed revision would exempt TBAc from the definition of VOC on the basis that this compound has negligible contribution to tropospheric ozone formation. The final rule is still pending. Implementation of this rule would probably result in increased TBAc usage.
The OSHA Permissible Exposure Limit (PEL) and ACGIH TLV for TBAc (8 h time-weighted) is 200 ppm (950 mg/m3). These standards are primarily based on exposed workers' eye and respiratory system irritation. TBA is genotoxic in an oxidative DNA damage-sensitive strain of Salmonella and in human HL-60 leukemia cells. Additionally, TBAc is metabolized to tert-butanol (TBA) in rats. TBA has been demonstrated to be carcinogenic in both rats and mice.
TBAc has been demonstrated to be substantially metabolized to tert-butanol (TBA) in rats, and a positive TBA genotoxicity study suggests that TBA may cause oxidative DNA damage. TBA has been shown to induce tumors in both rats and mice. The Office of Environmental Health Hazard Assessment has calculated an oral cancer potency factor (CSF) for TBA of 3 x 10(-3)(mg/kg-day)(-1)[4]. Therefore, TBAc should be considered to pose a potential cancer risk to humans because of the metabolic conversion to TBA. An acute 1-h reference exposure level of 1 mg/m3 can be calculated from the extrapolated no observed adverse effect level of 50 mg/m3.
References
[1] Charlotte E. Coomber . "Catalytic direct amidations in tert-butyl acetate using B(OCH2CF3)31." Organic & Biomolecular Chemistry 17 26 (2019): Pages 6465-6469.
[2] Nirmala Mohanta and Boopathy Gnanaprakasam. "Catalyst-Assisted Selective Vinylation and Methylallylation of a Quaternary Carbon Center Using tert-Butyl Acetate." The Journal of Organic Chemistry 88 14 (2023): 9686–9703.
[3] Scott W. Roberts and Oliver R. Thiel. "Mechanistic Insights and Safety Evaluation of the Ritter Reaction Utilizing tert-Butyl Acetate as the tert-Butyl Cation Source." Organic Process Research & Development 16 12 (2012): 2058–2063.
[4] Budroe, J. D. , et al. "Acute toxicity and cancer risk assessment values for tert-butyl acetate. " Regulatory Toxicology and Pharmacology: RTP 2(2004):40.
See also
Lastest Price from tert-Butyl acetate manufacturers
US $1.00/KG2025-04-21
- CAS:
- 540-88-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100mt
US $0.00/kg2025-04-15
- CAS:
- 540-88-5
- Min. Order:
- 20kg
- Purity:
- 99%
- Supply Ability:
- 20 tons