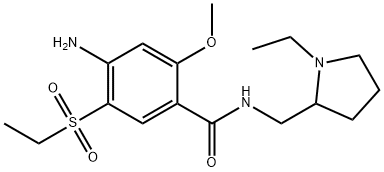
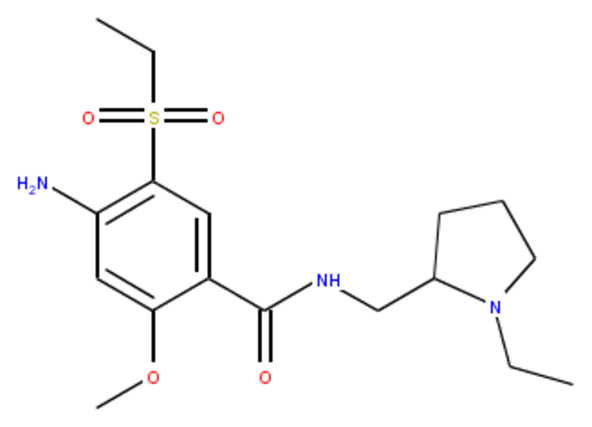
A dopamine antagonist: Amisulpride
Description
Amisulpride is a dopamine antagonist that has been used as an oral atypical antipsychotic in Europe since 1988. Compared to other dopamine antagonists, amisulpride possesses unique pharmacological properties that result in a more favorable safety profile. On February 27, 2020, intravenous amisulpride was approved by the US Food and Drug Administration (FDA) to prevent and treat PONV.
Mechanism of action
Amisulpride is a selective antagonist for dopamine-2 (D2) and dopamine-3 (D3) receptors, located in the area postrema and involved in emesis. When dopamine is released into the chemoreceptor trigger zone, D2 receptors stimulate the vomiting center. The antagonistic effect of amisulpride on dopamine receptors decreases the incidence of nausea and vomiting. The drug has low affinities for 5-HT2B and 5-HT7 receptors but no considerable affinity for other receptor types[1].
Uses
Amisulpride is a substituted benzamide derivative that selectively enhances dopaminergic neurotransmission; it is being developed as a potential treatment for the chronic mood disorder dysthymia[2]. As an atypical antipsychotic, it is used in the treatment of acute episodes of schizophrenia[3]. Amisulpride alleviates both positive and negative symptoms of schizophrenia, and it exhibits antidepressant properties in patients with psychiatric disorders, dysthymia, and major depression. Amisulpride and Paliperidone are two antipsychotics used to treat schizophrenia in the UK. Data from several double-blind trials indicate that amisulpride is effective in the medium-term treatment of dysthymia and is very well tolerated in most patients[4]. Further efficacy and tolerability comparisons with the selective serotonin (5-hydroxytryptamine; 5-HT) reuptake inhibitors would be beneficial.
This drug is a new drug for the management of postoperative nausea and vomiting[1]. In the US, the intravenous formulation of amisulpride is used to treat and prevent postoperative nausea and vomiting in adults, either as monotherapy or in combination with another antiemetic agent of a different drug class. In the research, for vomiting, antiemetic rescue, and nausea evaluated individually, the 5-mg dose of amisulpride (but not the 1-mg and 20-mg doses) showed statistically significant benefits when compared with placebo.
Side effects
Along with its needed effects, amisulpride may cause some unwanted effects. Although not all of these side effects may occur, if they do occur, they may need medical attention.
A. Amisulpride may cause serious side effects:
sudden dizziness (like you might pass out);
fast or pounding heartbeats, fluttering in your chest;
shortness of breath;
or low potassium level--leg cramps, constipation, irregular heartbeats, fluttering in your chest, increased thirst or urination, numbness or tingling, muscle weakness, or limp feeling.
B.Common side effects of amisulpride may include:
low potassium;
feeling light-headed;
stomach bloating; or
pain where the medicine was injected.
References
[1] Stacy L Haber, Ani Minasian, April Graybill. “Amisulpride: A New Drug for Management of Postoperative Nausea and Vomiting.” Annals of Pharmacotherapy 55 10 (2021): 1276–1282.
[2] S. Noble, P. Benfield. “Amisulpride: A Review of its Clinical Potential in Dysthymia.” CNS drugs 12 1 (1900): 471–483.
[3] A. Puech. “Amisulpride, an atypical antipsychotic, in the treatment of acute episodes of schizophrenia: a dose-ranging study vs. haloperidol.” Acta Psychiatrica Scandinavica 98 1 (2007): 65–72.
[4] Ali Abdall-Razak. “A cost-utility analysis of Amisulpride and Paliperidone in the treatment of Schizophrenia.” Heliyon (2019).
Related articles And Qustion
See also
Lastest Price from Amisulpride manufacturers
US $0.00/kg2025-09-28
- CAS:
- 71675-85-9
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $0.00-0.00/KG2025-04-21
- CAS:
- 71675-85-9
- Min. Order:
- 100g
- Purity:
- 99~101%
- Supply Ability:
- 200kg/month