5-Azacytidine: Mechanism of Action AND Mechanism of Resistance
What is 5-Azacytidine?
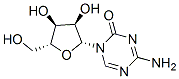
5-Azacytidine (NSC-102816), 4-amino-1-β-d-ribofuranosyl-1,3,5-triazine- 2(1H)-one, 5-azacitidine, C8H12N4O5, Mr 244.21, is a pyrimidine analog that was first isolated as a fermentation product from Streptoverticillium cultures and was chemically synthesized in Czechoslovakia in 1964. Structurally, 5-azacytidine differs from cytidine by the substitution of nitrogen in the 5 position of the pyrimidine nucleus.

Mechanism of Action
5-Azacytidine shares the facilitated transport system for cytidine for entry into cells and must be phosphorylated to exert cytotoxic effects. Conversion to the monophosphate is catalyzed by the enzyme uridine – cytidine kinase , and this is likely the rate-limiting step for drug activation. 5-Azacytidine monophosphate inhibits the enzyme orotidylate decarboxylase and interferes with de novo pyrimidine biosynthesis. Subsequent metabolism of the monophosphate to azacytidine di- and triphosphate, catalyzed by cytidine monophosphate kinase and nucleoside diphosphate kinase, occurs rapidly and does not appear to be a rate-limiting step in drug activation.
The diphosphate of azacytidine is a substrate for ribonucleotide reductase and dAzaCTP (deoxyribonucleotide triphosphate of azacytidine) for DNA polymerase, allowing direct incorporation of drug into DNA. This pathway may be critical for cytotoxicity because active DNA synthesis correlates with drug sensitivity in vitro. In addition, incorporation of 5- azacytidine into DNA may affect gene expression. Mammalian DNA contains ca. 5 % of incorporated cytosine methylated in the 5 position; methylation appears to inhibit gene expression. Thus the globin gene is hypomethylated in bone marrow as compared to other tissues. The DNA containing even low levels of 5-azacytidine is a potent inhibitor of the enzyme responsible for cytosine methylation, i.e., DNA cytosine methyltransferase. The enzyme inhibition is disproportionately great compared with the small amount of incorporated fraudulent base, and appears to result from formation of a stable complex between 5- azacytidine residues and the methyltransferase, similar to the complex formed with thymidylate synthase and FdUMP. Thus, in vitro treatment with 5-azacytidine can induce DNA hypomethylation and differentiation of murine cells. More recently these observations have been extended to clinical medicine. Azacytidine treatment in a patient with severe β- thalassemia could stimulate gamma-globin synthesis by inducing hypomethylation and expression of the gamma-globin gene.
After conversion to a triphosphate, azacytidine also competes with CTP for incorporation into RNA and inhibits maturation of ribosomal and transfer RNA. This causes disassembly of polyribosomes and interferes with protein synthesis.
Mechanism of Resistance
Cellular resistance to 5-azacytidine may be the result of either decreased drug activation or possibly increased drug degradation. Drug metabolism by cytidine kinase appears to be rate-limiting in drug activation; deletion of this enzyme has been reported in 5- azacytidineresistant cells in vitro. Cytidine deaminase degrades 5-azacytidine to 5- azauridine; however, the role of this enzyme in drug resistance remains uncertain. For example, drug toxicity may be dependent on deamination; bacteria incapable of forming 5-azauridine from 5-azacytidine are resistant to the drug.
You may like
Lastest Price from 5-Azacytidine manufacturers

US $2.00-5.00/kg2025-07-01
- CAS:
- 320-67-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg

US $5.00-0.50/KG2025-06-13
- CAS:
- 320-67-2
- Min. Order:
- 0.10000000149011612KG
- Purity:
- 99% hplc
- Supply Ability:
- 5000kg