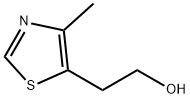
5-(2-Hydroxyethyl)-4-methylthiazole: Applications in Medicinal Chemistry and its Synthesis Method
General Description
5-(2-Hydroxyethyl)-4-methylthiazole, also known as 4-Methyl-5-thiazoleethanol (MTE), is a bacterial metabolite that exists in both Escherichia coli and B. pseudomallei. MTE also exists in plants and can be isolated from the ether-soluble alkaloids of Panax notoginseng. MTE is widely used in the field of biological metabolomics and can be used as a new biomarker for B. pseudomallei.

Figure 1. 5-(2-Hydroxyethyl)-4-methylthiazole
Applications
4-Methyl-5-thiazolylethanol (MTE) was covalently attached to graphene oxide (GO) to prepare a new composite material with excellent adsorption selectivity for copper ions (Cu2+). The introduction of MTE effectively inactivates most of the carboxyl groups of GO, and the covalently-linked thiazole ring with N- and S-containing heteroatoms endows GO-MTE composite with excellent adsorption selectivity for Cu2+ in aqueous solution. The adsorption capacities of GO-MTE composite for Cu2+ are 2.39, 17.08 and 26.64 times higher than those of Pb2+, Nd3+ and Y3+, respectively, indicating that it possesses stronger binding ability for Cu2+. After eight adsorption/desorption cycles, the recycled GO-MTE composite still maintained high adsorption capacity and removal rate for Cu2+, indicating that GO-MTE composite possesses excellent stability and reusability, and it could be used for the separation and recovery of Cu2+ from aqueous solutions.
Studies have shown that 4-methyl-5-thiazoleethanol (MTE) is a degradation product of thiamine (vitamin B1), and MTE has also been found in the culture supernatant of B. pseudomallei. Burkholderia pseudomallei is an emerging pathogen that causes melioidosis, a serious and potentially fatal disease which requires prolonged antibiotics to prevent relapse. MTE can be used as a new biomarker for B. pseudomallei and for the development of related drugs.
Synthesis Method
5-(2-Hydroxyethyl)-4-methylthiazole was obtained by preferentially preparing α-acetyl-γ-butyrolactone intermediate using sulfonyl chloride as raw material, and then by vacuum distillation. The specific steps are as follows:
In a sealed reactor equipped with a stirring and cooling device, 80 parts by weight of sulfuryl chloride are slowly added to treat 100 parts by weight of alpha-acetyl-gamma-butyrolactone while maintaining the temperature between 40-42°C for 1.5-2.5 hours. After the reaction is completed, the mixture is dried with calcium chloride (CaCl2) and then filtered to obtain refined alpha-acetyl-alpha-butyrolactone.
The alpha-acetyl-gamma-butyrolactone obtained earlier is reintroduced into a sealed reactor with a condensing reflux device. 77 parts by weight of 5% sulfuric acid or 160 parts by weight of 5% hydrochloric acid are added, and the mixture is heated to boiling point and refluxed for 5-7 hours. The reaction mixture is then extracted with dichloromethane, and the dichloromethane extract of 3-chloro-3-acetylpropanol is collected. In the subsequent step, the extract is mixed with 100 parts by weight of formamide in a reactor, the reactor temperature is maintained between 28-32°C, and then 54 parts by weight of phosphorus pentasulfide are slowly added over 1.5-2 hours. After further stirring and extraction, the thioformamide dichloromethane solution is mixed with the previous 3-chloro-3-acetylpropanol solution and refluxed for 5-6 hours. The resulting mixture is cooled, the pH value is adjusted to 9-10 with a 5% NaOH solution, the oil phase is separated and further extracted with dichloromethane. The final product, 5-(2-hydroxyethyl)-4-methylthiazole, is then obtained by vacuum distillation.
References:
[1] SERGIO A. CALDARELLI. New Bis-thiazolium Analogues as Potential Antimalarial Agents: Design, Synthesis, and Biological Evaluation[J]. Journal of Medicinal Chemistry, 2013, 56 2: 387-592. DOI:10.1021/jm3014585.[2] TAKAYOSHI SUZUKI*. Rapid Discovery of Highly Potent and Selective Inhibitors of Histone Deacetylase 8 Using Click Chemistry to Generate Candidate Libraries[J]. Journal of Medicinal Chemistry, 2012, 55 22: 9363-10316. DOI:10.1021/jm300837y.
See also
Lastest Price from 5-(2-Hydroxyethyl)-4-methylthiazole manufacturers

US $0.00-0.00/KG2025-06-26
- CAS:
- 137-00-8
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 10000KGS

US $0.00/kg2025-06-13
- CAS:
- 137-00-8
- Min. Order:
- 25kg
- Purity:
- 99.5%-99.9%
- Supply Ability:
- 200Tons