4-tert-Butylthiophenol: properties, applications and safety
General Description
4-tert-Butylthiophenol is an organic compound with unique properties that make it valuable in various applications. It has a thiophene ring attached to a tert-butyl group and a hydroxyl group, giving it stability and reactivity. The compound is sparingly soluble in water but dissolves well in organic solvents. Its distinct odor makes it important in fragrance development and odorant additives. 4-tert-Butylthiophenol can undergo esterification, oxidation, and substitution reactions, making it versatile for organic synthesis. However, it may degrade when exposed to oxidizing agents or prolonged exposure to air and light. Its applications include agrochemicals, pharmaceuticals, fragrances, rubber accelerators, and polymer stabilizers. Safety precautions must be taken when handling this compound to prevent health risks.

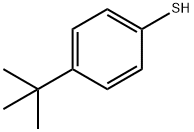
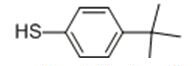
Figure 1. 4-tert-Butylthiophenol
Properties
4-tert-Butylthiophenol is an organic compound with a unique set of properties that make it useful in various applications. Its molecular structure consists of a thiophene ring attached to a tert-butyl group and a hydroxyl group, which imparts stability and reactivity to the compound. It is sparingly soluble in water but dissolves well in organic solvents like ethanol, methanol, and ether. The distinct thiol-like odor of 4-tert-Butylthiophenol makes it important in certain applications, such as odorant additives and fragrance development. Chemically, 4-tert-Butylthiophenol can undergo esterification, oxidation, and substitution reactions, making it versatile for use in organic synthesis. However, it may degrade upon exposure to strong oxidizing agents or prolonged exposure to air and light. Overall, the properties of 4-tert-Butylthiophenol make it valuable in the fields of organic synthesis, fragrance production, and chemical research. This compound has many applications in the industry, including as an intermediate in the manufacture of agrochemicals, pharmaceuticals, and fragrances. It is also used as a flavoring agent in food and beverages. In addition, 4-tert-Butylthiophenol is used in the production of rubber accelerators and polymer stabilizers. In conclusion, 4-tert-Butylthiophenol is an important compound with unique properties that make it useful in various applications. Its versatility in organic synthesis, distinctive odor, and solubility are just a few of the qualities that make it a valuable compound in the chemical industry. 1
Applications
4-tert-Butylthiophenol is a compound that has various applications in the field of photochemistry and electrochemistry. In a study, it is used in conjunction with visible light to convert quinones to quinols, mimicking the function of quinone pools in photosynthesis. This highlights its potential role in harnessing light energy for chemical reactions. In other study, 4-tert-butylthiophenol is compared to another compound, 1-adamantanethiolate, in the context of electrochemiluminescence (ECL) of metal nanoclusters. The results show that the presence of 4-tert-butylthiophenol weakens the ECL signal of the nanocluster. Additionally, the compound exhibits solid-state ECL emissions at a wavelength of 770 nm, which is blue-shifted compared to its corresponding solid-state photoluminescence emission. These findings demonstrate that the properties and performance of metal nanoclusters can be significantly influenced by the subtle differences in the composition of their core. The use of 4-tert-butylthiophenol and similar compounds in atomically precise emissive metal nanoclusters shows promise for applications in sensing and immunoassay platforms, offering a potential basis for developing advanced diagnostic tools. 2,3
Safety
4-tert-Butylthiopheno presents safety concerns that should be carefully considered. Structurally similar to tyrosine, it serves as a substrate for tyrosinase, which oxidizes it to form a quinone. This quinone can rapidly react with glutathione and, in the absence of a sufficient GSH defense system, may generate reactive oxygen species that can damage melanocytes, leading to leukoderma/vitiligo. Additionally, it has been found to be irritating to the eyes, nose, and throat, and inhalation can lead to difficulty breathing. In the event of a spill, it is important to use absorbent paper to pick up all liquid material and seal contaminated clothing and absorbent paper in a vapor-tight plastic bag for proper disposal. Contaminated surfaces should be solvent-washed with alcohol followed by a strong soap and water solution. It is crucial not to reenter the contaminated area until it has been verified as properly cleaned by the Safety Officer or another responsible person. Overall, handling 4-tert-butylthiophenol requires strict adherence to safety protocols to prevent exposure and minimize potential health risks. 4
Reference
1. PubChem. COMPOUND SUMMARY: 4-tert-Butylphenol. National Library of Medicine, PubChem CID: 7393.
2. Chen S, Liu Y, Kuang K, Yin B, Wang X, Jiang L, Wang P, Pei Y, Zhu M. Impact of the metal core on the electrochemiluminescence of a pair of atomically precise Au20 nanocluster isomers. Commun Chem. 2023 May 31;6(1):105.
3. Nagata T, Kikuzawa Y. An approach towards artificial quinone pools by use of photo- and redox-active dendritic molecules. Biochim Biophys Acta. 2007 Jun;1767(6):648-652.
4. 4-tert-Butylphenol. Toxin and Toxin Target Database, T3D4881.
Related articles And Qustion
See also
Lastest Price from 4-TERT-BUTYLTHIOPHENOL manufacturers

US $1786.00/Kg2024-09-06
- CAS:
- 2396-68-1
- Min. Order:
- 1Kg
- Purity:
- 98
- Supply Ability:
- 500 Kg

US $60.00-600.00/kg2024-08-27
- CAS:
- 2396-68-1
- Min. Order:
- 10kg
- Purity:
- 0.99
- Supply Ability:
- 20tons