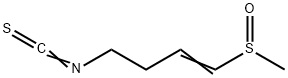
Sulforaphene: pharmacokinetics, activities and safety
General Description
Sulforaphene is a natural compound found in cruciferous vegetables like broccoli and cauliflower. It undergoes complex metabolic pathways in mammalian cells and has been extensively studied in terms of its pharmacokinetics. Sulforaphene is rapidly absorbed after oral administration, reaching peak concentration within one hour. Its half-life ranges from 1.77-1.9 hours in humans. It can cross the placental barrier, as evidenced by its detection in embryos. Sulforaphene exhibits potential health benefits, including protective effects against cancer and cardiovascular disease. However, caution should be exercised with high doses or concentrated supplements, as they may have adverse effects on the liver and kidneys. Further research is needed to fully understand its mechanisms of action and effectiveness in various medical applications.

Figure 1. Capsules of sulforaphene
Pharmacokinetics
Sulforaphene is a natural compound found in cruciferous vegetables, such as broccoli and cauliflower. Upon entering mammalian cells, sulforaphene undergoes a reaction with glutathione (GSH) in the presence of glutathione-S-transferase (GST), which leads to the formation of the glutathione conjugate of sulforaphene. This conjugate then undergoes sequential conversions catalyzed by γ-glutamyltranspeptidase (γGT), cysteinylglycinase (CGase), and N-acetyltransferase (NAT), resulting in the production of the N-acetylcysteine conjugate of sulforaphene. Sulforaphene and its metabolites can be quantified collectively by cyclocondensation with 1,2-benzenedithiol, with sensitivity in the picomolar range. This method has been widely used to measure the levels of sulforaphene and its metabolites in blood, plasma, urine, and tissues following sulforaphene administration to rodents and humans. Pharmacokinetic studies have shown that sulforaphene is rapidly absorbed following oral administration, and the concentration of dithiocarbamates in the plasma increases rapidly, reaching a peak (Cmax) of up to 60 µM 1 hour after dosing. The half-life of sulforaphene in rats is approximately 6.7 hours, while in humans it ranges from 1.77-1.9 hours. The clearance of sulforaphene in humans is approximately 369 ± 53 mL/min. Sulforaphene crosses the placental barrier, and its metabolites have been detected in embryos 2 hours post-treatment of pregnant mice with a single dose of sulforaphene. In conclusion, sulforaphene undergoes complex metabolic pathways upon entering mammalian cells, and its pharmacokinetics have been extensively studied in both rodents and humans. The use of analytical tools, such as mass spectrometry, has allowed for sensitive and specific analysis of sulforaphene and its metabolites in various biological samples. 1
Activities
Sulforaphene is a potent isothiocyanate compound found in cruciferous vegetables like cabbage, cauliflower, and kale. It exists in its stored form as glucoraphanin and is particularly abundant in broccoli, especially in broccoli sprouts. The conversion of glucoraphanin into sulforaphene requires the plant enzyme myrosinase. Once consumed, sulforaphene undergoes metabolism through the mercapturic acid pathway. It is conjugated with glutathione and undergoes further biotransformation, resulting in the production of various metabolites. Extensive research has been conducted on sulforaphene, primarily due to its potential health benefits. Studies have indicated that sulforaphene exhibits protective effects against different types of cancer. Additionally, it has been suggested that sulforaphene can reduce the risk of cardiovascular disease. Furthermore, it may also have positive effects in the treatment of autism and osteoporosis. In our review, we provide a concise summary of the intriguing properties of sulforaphene. We discuss both in vitro and in vivo methods/models, as well as clinical studies that have explored the therapeutic potential of this compound. Please note that while there is promising research surrounding sulforaphene, further studies are still required to fully understand its mechanisms of action and establish its effectiveness in various medical applications. 2
Safety
Sulforaphene has been studied for its potential health benefits. However, when considering its toxicity, research has shown that sulforaphene exhibits low acute toxicity and is generally well-tolerated at moderate doses. In animal studies, high doses of sulforaphene have been associated with adverse effects on the liver and kidneys, indicating a potential for toxicity at elevated levels. Furthermore, while human studies are limited, it is essential to approach sulforaphene consumption with caution, particularly in high doses or as a concentrated supplement, due to its potential for adverse effects on certain organs. 3
Reference
1. Yagishita Y, Fahey JW, Dinkova-Kostova AT, Kensler TW. Broccoli or Sulforaphane: Is It the Source or Dose That Matters? Molecules. 2019 Oct 6;24(19):3593.
2. Vanduchova A, Anzenbacher P, Anzenbacherova E. Isothiocyanate from Broccoli, Sulforaphane, and Its Properties. J Med Food. 2019 Feb;22(2):121-126.
3. PubChem. COMPOUND SUMMARY: Sulforaphene. National Library of Medicine, PubChem CID: 6433206.
Related articles And Qustion
See also
Lastest Price from Sulforaphene manufacturers

US $0.00/kg2025-04-02
- CAS:
- 592-95-0
- Min. Order:
- 1kg
- Purity:
- >98%
- Supply Ability:
- 1000 kg
US $0.00/mg2023-02-24
- CAS:
- 592-95-0
- Min. Order:
- 20mg
- Purity:
- ≥98%(HPLC)
- Supply Ability:
- 10 g