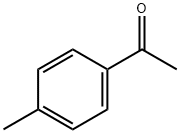
4'-Methylacetophenone: Applications in Medicinal chemistry and its Health Hazards
4′-Methylacetophenone (4-MACP), a sweet, acetophenone, and bitter almond tasting compound is used as a flavoring agent. It reacts with morpholine to get 4-(p-tolyl-thioacetyl)-morpholine in the presence of sulfur as a reagent.
Applications
4'-Methylacetophenone has been detected, but not quantified, in several different foods, such as citrus, garden tomato, pepper (spice), garden tomato (var.), and milk and milk products. This could make 4'-methylacetophenone a potential biomarker for the consumption of these foods.

4-Methylacetophenone is used for blossom notes in mimosa and for the production of hawthorn type perfumes, especially in soap perfumes. Further, it is used as an intermediate in the manufacture of active pharmaceutical ingredients, perfumes and cosmetics. Conventionally, 4-MACP is prepared from toluene and acetic anhydride or acetyl chloride by a Fridel–Crafts catalyst AlCl3.
Health Hazards
Eye: May cause eye irritation.
Skin: May cause skin irritation.
Ingestion: 4'-Methylacetophenone May cause gastrointestinal irritation with nausea, vomiting and diarrhea. May be harmful if swallowed.
Inhalation: 4'-Methylacetophenone May cause respiratory tract irritation. Material has a very low vapor pressure at room temperature, so inhalation exposures are not expected unless material is heated or misted.
Chronic: Prolonged or repeated skin contact may cause defatting and dermatitis.
References:
[1] T JAIMOL A P S A K Pandey. Selective acetylation of toluene to 4-methylacetophenone over zeolite catalysts[J]. Journal of Molecular Catalysis A: Chemical, 2001, 170 1: 1-292. DOI:10.1016/S1381-1169(00)00569-0.Related articles And Qustion
Lastest Price from 4'-Methylacetophenone manufacturers

US $10.00/ASSAYS2025-05-04
- CAS:
- 122-00-9
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 10 ton

US $20.00/KG2025-04-21
- CAS:
- 122-00-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 300 Tons/ year