4,4-Piperidinediol hydrochloride: synthesis and applications in organic synthesis
General Description
The synthesis of 4,4-Piperidinediol hydrochloride involves a sequence of C-H and C-C bond functionalizations starting from phenyl(2-piperidinyl)methanone hydrochloride. Inspired by the Norrish-Yang Type II reaction, α-hydroxy-β-lactams are generated from α-ketoamide derivatives under visible light conditions, followed by selective cleavage of the C(sp2)-C(sp3) bond using a Rh-complex. This leads to the formation of α-acyl intermediates, which undergo sequential reactions to produce N-fused bicycles, including indolizidines. Computational studies have elucidated the positional selectivity of C-C cleavage. Furthermore, 4,4-Piperidinediol hydrochloride serves as a crucial building block for bis-bispidine tetraazamacrocycles, offering versatility in incorporating different functional groups. It also plays a key role in the synthesis of pharmaceutical compounds, demonstrating its significance in the pharmaceutical industry. Overall, this compound enables the efficient construction of complex molecules with diverse applications in organic synthesis and pharmaceuticals.

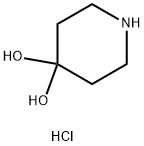
Figure 1. 4,4-Piperidinediol hydrochloride
Synthesis
The synthesis of 4,4-Piperidinediol hydrochloride involves a series of sequential C-H and C-C bond functionalizations starting from phenyl(2-piperidinyl)methanone hydrochloride. By drawing inspiration from the Norrish-Yang Type II reaction, the C-H functionalization of azacycles is achieved by generating α-hydroxy-β-lactams (e.g., compound II) from precursor α-ketoamide derivatives RC(O)C(O)C6H5 (where R can be piperidin-1-yl, morpholin-4-yl, 3-azaspiro[5.5]undecan-3-yl, etc.) under mild, visible light conditions. Subsequently, a selective cleavage of the distal C(sp2)-C(sp3) bond in α-hydroxy-β-lactams (e.g., II) is carried out using a Rh-complex, leading to the formation of α-acyl intermediates. These intermediates undergo sequential Rh-catalyzed decarbonylation, 1,4-addition to an electrophile, and aldol cyclization reactions, resulting in the synthesis of N-fused bicycles, including indolizidines. Computational studies have provided valuable insights into the mechanisms underlying the positional selectivity of C-C cleavage, which is strongly influenced by the groups bound to Rh trans to the phosphine ligand. Overall, this synthetic approach enables the efficient construction of substituted indolizidines and related N-fused bicycles, exemplified by compounds like 1R,2S,8aS/1R,2R,8aS-I, starting from the readily available phenyl(2-piperidinyl)methanone hydrochloride precursor. 1
Applications in organic synthesis
Building block
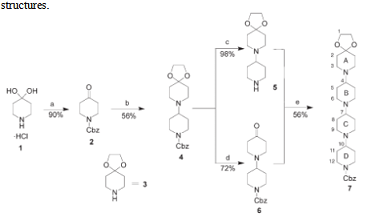
4,4-Piperidinediol hydrochloride is a versatile compound with various applications. One of its significant uses lies in the field of organic synthesis. 4,4-Piperidinediol hydrochloride serves as an essential building block for the construction of highly rigid and preorganized bis-bispidine tetraazamacrocycles. The synthesis of these tetraazamacrocycles involves utilizing N-Boc-N'-allylbispidinone as a starting material. The modular approach employed in this synthesis enables the independent incorporation of different functional groups into the macrocyclic framework. Additionally, selective derivatization of one functionality can be achieved in the presence of another. These bis-bispidine tetraazamacrocycles have found applications in coordination chemistry and catalysis. Due to their rigidity and preorganization, they exhibit enhanced stability and selectivity in various chemical reactions. The incorporation of different functionalities allows for fine-tuning of their properties and applications in different fields. Overall, the synthesis of bis-bispidine tetraazamacrocycles using 4,4-Piperidinediol hydrochloride as a key building block offers a versatile and efficient method for the preparation of structurally complex molecules with diverse applications in chemistry and related disciplines. 2
Synthesis of pharmaceutical compounds
4,4-Piperidinediol hydrochloride plays a crucial role in the synthesis of pharmaceutical compounds with the general formula I [R1 is Me, CF3, amine, piperidinyl; R2 is amine; R3 is Me, CF3, F, Cl, Br], their pharmaceutically acceptable salts, and solvates. For instance, Compound I (where R1 = Cl, R2 = NH2, R3 = CF3) is prepared using 4,4-Piperidinediol hydrochloride as a key component in the synthetic process. The synthesis involves several steps, including the condensation of 4-amino-3-chloro-5-trifluoromethylbenzaldehyde with Me methoxyacetetate to produce (Z)-3-(4-amino-3-chloro-5-trifluoromethylphenyl)-2-methoxyacrylic acid. Subsequent demethylation and asymmetric reduction lead to the formation of (R)-3-(4-amino-3-chloro-5-trifluoromethylphenyl)-2-hydroxypropanoic acid, which then undergoes coupling with 1-(1H-imidazol-1-ylcarbonyl)-4-(1,2,4,5-tetrahydro-2-oxo-3H-1,3-benzodiazepin-3-yl)piperidine to yield Compound I. Furthermore, Compound I can be further modified into corresponding propanamide by amidation with cyclic amines. This versatile synthetic pathway demonstrates the pivotal role of 4,4-Piperidinediol hydrochloride in the preparation of pharmaceutical compounds, showcasing its significance in the pharmaceutical industry. 3
Reference
1. Ham JS, Park B, Son M, Roque JB, Jurczyk J, Yeung CS, Baik MH, Sarpong R. C-H/C-C Functionalization Approach to N-Fused Heterocycles from Saturated Azacycles. J Am Chem Soc. 2020 Jul 29;142(30):13041-13050.
2. Wang Z, Miller EJ, Scalia SJ. Modular synthesis of functionalized bis-bispidine tetraazamacrocycles. Org Lett. 2011 Dec 16;13(24):6540-6543.
3. Novel preparation process of benzodiazepinyl derivatives using asymmetric reduction. 2009, Patent Number: WO2009021942.
Related articles And Qustion
See also
Lastest Price from 4,4-Piperidinediol hydrochloride manufacturers

US $10.00/kg2024-05-30
- CAS:
- 40064-34-4
- Min. Order:
- 0.1kg
- Purity:
- 99%
- Supply Ability:
- 20ton