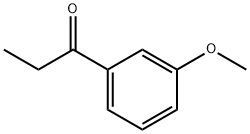
3'-methoxypropiophenone: Synthesis and Applications in Pharmaceutical Chemistry
General Description
3'-methoxypropiophenone, with the CAS number 37951-49-8, is an organic compound belonging to the ketone class of compounds. It is a yellow to yellow brown liquid with a boiling point of 259 °C. 3'-methoxypropiophenone has a wide range of applications in the fields of medicine, chemistry, and industry. 3'-methoxypropiophenone is used as an intermediate in the synthesis of pharmaceuticals.

Figure 1. Properties of 3'-methoxypropiophenone
Preparation
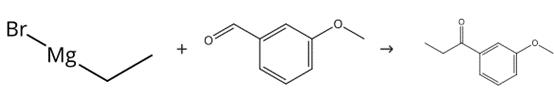
Figure 2. Preparation of 3'-methoxypropiophenone
To a stirred solution of 3-(methyloxy)benzaldehyde (0.895 mL, 7.34 mmol) in diethyl ether (10 mL) cooled in an ice bath was added ethylmagnesium bromide (1.0M solution in THF) (11.02 mL, 11.02 mmol), the solution was stirred for 3 h in an ice bath, after which aqueous HCl (0.6M) (20 mL) was added. The aqueous layer was then extracted with DCM (4 x 30 mL), the combined organic extracts were dried over a phase separating column and concentrated. The residue was used in the next step without any further purification. To a solution of oxalyl chloride (0.964 mL, 11.02 mmol) in DCM (4 mL) cooled to -78°C was added DMSO (1.042 mL, 14.69 mmol) dropwise, followed by a solution of the material isolated in the first step in DCM (6 mL). The resulting mixture was 20 stirred for 1 h at -78°C before the addition of triethylamine (5.12 mL, 36.7 mmol), after which the reaction mixture was stirred at -78°C for 1 h. The reaction was quenched at - 78°C by water (20 mL). The aqueous layer was then extracted with DCM (3 x 50 mL), the combined organic extracts were dried over a phase separating column and concentrated. Purification by chromatography on silica gel, eluting with a gradient of 0-25% ethyl acetate in cyclohexane, afforded the title compound 3'-methoxypropiophenone (1.126 g).1
Application
Intermediate in Preparation of Tapentadol
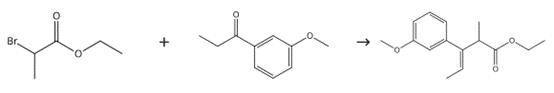
Figure 3. Intermediate in Preparation of Tapentadol
Tetrahydrofuran (200 ml) and zinc metal (100 g) were taken under nitrogen atmosphere, the mixture was heated at 65±2°C, followed by the addition of ethyl 2-bromopropionate (20 g) and then drop-wise addition of trimethylsilyl chloride (10 ml). A mixture of 3'-methoxypropiophenone (100 g), ethyl 2-bromopropionate (120 g) and tetrahydrofuran (300 ml) was added to the above mixture while maintaining the temperature at 65-75 °C. After complete addition of the above mixture, the reaction mass was refluxed for 1-2 hours and then cooled to 0-5°C, followed by the addition of 15% dilute HCl solution (200 ml) at below 20°C. Toluene (300 ml) was added to the resulting mixture and then stirred for 30 minutes. The reaction mass was transferred into a separating funnel and then allowed to settle for 10-15 minutes, followed by the separation of the upper organic layer. The resulting aqueous layer was separated and then placed into a reaction flask. Toluene (200 ml) was added to the aqueous layer and the reaction mixture was stirred for 30 minutes, followed by transferring into the separating funnel and allowing it to settle for 10-15 minutes. The upper organic layer was separated. The entire extracted organic layer was combined and then washed with saturated sodium chloride solution (200 ml). The organic layer was dried over anhydrous sodium sulphate (50 g), followed by the distillation of solvents completely at 55°C and further applying high vacuum (5mm/Hg) at 55°C for 5-7 hours. Yield: 237 g (100%).2
Intermediate in Synthesis of 2,3,6-Trisubstituted Pyridines
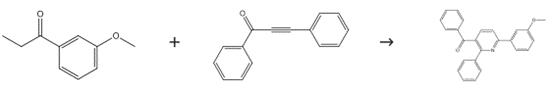
Figure 4. Intermediate in Synthesis of 2,3,6-Trisubstituted Pyridines
Dissolve a mixture of 1,3-diphenylprop-2-yn-1-one (0.2 mmol), 3'-methoxypropiophenone (0.4 mmol), ammonium carbonate (4.0 equivalents), Cu(OAc)2 (10 mol %) and 2,2,6,6-tetramethylpiperidine-N-oxyl (2.0 equivalents) in dimethyl sulfoxide (1 mL). Stir the reaction mixture at 120°C (oil bath temperature) for 20 hours. Cool the reaction mixture to room temperature. Extract the solvent with ethyl acetate. Wash the resulting mixture with brine. Dry the mixture with Na2SO4. Evaporate the solvent in vacuo. Purify the residue by column chromatography, eluting with petroleum ether/ethyl acetate (40:1).3
References
1. WO2011075559 A1 2011-06-23.
2. WO2012146978 A2 2012-11-01.
3. Zhan, Zhen Z.; et al. ChemistrySelect (2021), 6(17), 4160-4165
You may like
Related articles And Qustion
Lastest Price from 3'-methoxypropiophenone manufacturers

US $30.00-10.00/KG2025-04-15
- CAS:
- 37951-49-8
- Min. Order:
- 50KG
- Purity:
- 99%
- Supply Ability:
- 500000kg

US $0.00-0.00/kg2025-04-04
- CAS:
- 37951-49-8
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton