2-Octanone:Smell,Refractive Index,Preparation and Reaction
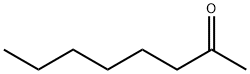
2-Octanone is a colorless volatile liquid with the formula CH3C(O)C6H13. It is produced commercially for use in the fragrance industry.

Smell
2-Octanone has a floral and bitter, green, fruity (unripe apple) odor.
Refractive Index
The refractive Index of 2-Octanonen is 20/D 1.416 (lit.).
Preparation
2-Octanone is produced by the condensation of acetone and pentanal followed by hydrogenation of the alkene. It can also be produced by selective oxidation of 1-octene.
Reaction
Oxidation of 2-octanol
The nitric acid oxidation of 2-octanol to 2-octanone followed by further oxidation of 2-octanone to carboxylic acids was studied by (van Woezik & Westerterp 2000 and 2001). The oxidation of 2-octanol is carried out in a two-phase reaction system: an organic liquid phase, which initially contains 2-octanol, in contact with an aqueous nitric acid phase in which the reactions takes place. The reaction can be described with the following equations:a
where A is 2-octanol, P is 2-octanone, X are the further oxidation products and B is the nitrosonium ion, which also causes an autocatalytic behavior. The reaction is carried out in a semi-batch reactor in which aqueous nitric acid is initially present, and the organic component 2-octanol (A) is added at a constant feed rate until a desired molar ratio of the reactants is reached. The 2-octanol reacts to form 2-octanone and carboxylic acid. The heat of reaction is removed by a coolant, which flows through an external jacket.
Under normal operating conditions, when the temperature in the reactor does not exceed the limit of approximately 0 °C throughout the reaction, only a very small fraction (about 7.5 %) of the 2-octanone is converted to carboxylic acids. However, if the temperature at any time exceeds approximately 5 °C, runaway conditions develop, which may lead to a maximal temperature of over 200 °C, and conversion of essentially all of the 2-octanone to carboxylic acid.1
Safety
Poison by ingestion. Moderately toxic by intraperitoned route. A sktn irritant. Flammable liquid when exposed to heat, flame, or oxidizers. To fight fire, use foam, alcohol foam. When heated to decomposition it emits acrid smoke and irritating fumes. See also ETHER and KETONES.
References:
[1] SHACHAM M, BRAUNER N. Considering parameter uncertainties in the design of safe processes[J]. Computer-aided chemical engineering, 1900, 16 1: 359-370. DOI:10.1016/B978-0-444-63433-7.50056-0.You may like
See also
Lastest Price from 2-Octanone manufacturers
US $0.00-0.00/KG2025-11-21
- CAS:
- 111-13-7
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $10.00/kg2025-04-21
- CAS:
- 111-13-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt