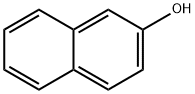
2-Naphthol:Production,Human Health,Environment
2-Naphthol is a fluorescent colorless (or occasionally yellow) crystalline solid. It is an isomer of 1-naphthol, differing by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol, but more reactive.
Production
2-Naphthol is produced by sulfonation of naphthalene to 2-naphthalene sulfonic acid, and reaction with caustic soda solution to obtain the sodium salt. Reaction of the sodium salt with sodium hydroxide and treating the melt with sulfuric acid yields 2-naphthol.
Uses
2-Naphthol is mainly used as intermediate for the production of dyestuffs. Further products are pharmaceuticals, fungicides, insecticides and odor agents. It is not quite clear whether 2-naphthol in these applications serves also as an intermediate or may directly be contained in these products. It is assumed that the main part is used as intermediate. The substance is also used as an antioxidant for rubber and plastic, grease and lubricants (ECB, 2000).
Human Health
2-Naphthol can be absorbed through the skin. Rapid conjugation with glucuronide and sulphate in the liver and renal excretion of the unchanged and conjugated forms seems to be the principal mechanism of elimination. The acute oral LD50 in rats was determined as 1320 mg/kg bw in a study following OECD TG 401.
Clinical signs included reduced activity, accelerated breathing, closure of eyes, nasal discharge and diarrhoea, and at exposure levels near to or exceeding the LD50 also tumbling, reduced reflexes and seizures. The inhalation 4-hour-LC50 in rats was determined as 2200 mg/m3 (aerosol; OECD TG 403).
Environment
2-Naphthol has a water solubility of 0.6 - 0.8 g/l, a vapor pressure of 1.4 Pa and a measured log Kow in the range of 2.01 – 2.84. 2-Naphthol is readily biodegradable as shown in a MITI test according to OECD 301C with non-adapted inoculum. A biodegradation of 68 % after 14 days was found.
There is no information on the degradation kinetic. The measured log Kow in the range of 2.01 to 2.84 does not indicate a significant potential for bio- or geoaccumulation. With a fugacity model (Mackay I) the following distribution can be predicted: hydrosphere: 83 %, atmosphere: 8 %, soil: 4.5 % and sediment: 4.5 %. The hydrosphere is therefore the target compartment for this substance. In water solution, photodegradation has been observed, but the half-life under environmental conditions was not estimated. The calculated half-life due to photochemical-oxidative degradation in the atmosphere by OH-radicals is about 2 hours.
Related articles And Qustion
Lastest Price from 2-Naphthol manufacturers

US $0.00/kg2025-09-02
- CAS:
- 135-19-3
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons

US $19.90/kg2025-04-21
- CAS:
- 135-19-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt