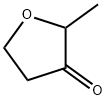
2-Methyltetrahydrofuran-3-one: Cosolvent for Sugar Esters & RIFM Safety Profile
2-Methyltetrahydrofuran-3-one has a bread, buttery topnote. May be prepared by hydrolytic decarboxylation of 2-methyl-4-carbomethoxytetrahydrofuran-3-one; by acid-catalyzed ring closure of p-alkoxy- diazoketones. When kept in the right conditions 2-Methyltetrahydrofuran-3-One stays fresh for about two to three years from the date of purchase. Past that point it will not turn toxic, yet the scent can flatten and pick up burnt off notes that blunt its charm. Refrigeration is helpful but not required. A cool cupboard away from direct light and strong heat sources is normally fine. The bottle should stay tightly closed and upright. Use polycone caps for stock solutions because the cone forms a snug seal that limits air exchange. Skip dropper tops as they let vapor escape and oxygen sneak in.

Phase Equilibrium and Thermodynamic Modeling of 2-Methyltetrahydrofuran-3-one
Recently, awareness of the importance of sustainable industrial processes and more natural foods has increased significantly. Within this context, sugar esters have stood out due to their renewable and ecological characteristics. Representing a significant part of the global market, these natural surfactants are derived from renewable sources, such as glucose extracted from raw materials such as sugar cane and corn. However, sugar ester production in supercritical media faces the challenge of low substrate solubility. To overcome this limitation, one strategy adopted is the incorporation of cosolvents such as 2-methyltetrahydrofuran-3-one (MeTHF-3-one), recognized for its effectiveness in supercritical environments. Widely used in the food industry and considered safe (GRAS), MeTHF-3-one improves the solubility of substrates, optimizing the interaction between the enzyme and reagents. Very recently, Barros et al. studied the phase behavior of the binary system CO2 (1) + MeTHF-3-one (2) and the ternary system containing CO2 (1) + MeTHF-3-one (2) + glucose (3). In this work, which was strategically designed to target future sugar ester synthesis at high pressures, it was observed that VLE with BP and DP transitions represented the phase behavior of the binary system CO2 + 2-Methyltetrahydrofuran-3-one. Additionally, it was noted that vapor–liquid equilibrium (VLE) in the presence or absence of a solid phase characterized the ternary system CO2 + MeTHF-3-one + glucose for two solute concentrations in MeTHF-3-one.[1]
To perform thermodynamic modeling, it was essential to determine three sets of binary interaction parameters. One set was intended for the system (CO2 (1) + MeTHF-3-one (2)) (k12 and l12), another for the system (CO2 (1) + lauric acid (3)) (k13 and l13), and the third for the system (2-Methyltetrahydrofuran-3-one (2) + lauric acid (3)) (k23 and l23). Obtaining each set of parameters involved fitting the thermodynamic model (PR-EoS) to the experimental data. To adjust (k12 and l12), experimental equilibrium (liquid + vapor) data (bubble points) from this study were used. The concentrations of lauric acid analyzed did not influence the transition pressures compared to the binary system consisting of CO2 and 2-Methyltetrahydrofuran-3-one. MeTHF-3-one has proven to be an effective cosolvent in enhancing the solubility of carbon dioxide and lauric acid systems. The systems investigated presented LCST behavior, with phase transition pressures increasing with increasing temperature, as observed for all studied systems. The PR-EoS model with the van der Waals quadratic mixing rule satisfactorily represented the observed phase behavior for all systems. The experimental results provide valuable insights for conducting enzymatic reactions to synthesize sugar esters in sc-CO2.
RIFM fragrance ingredient safety assessment, 2-methyltetrahydrofuran-3-one
2-Methyltetrahydrofuran-3-one was evaluated for genotoxicity, repeated dose toxicity, reproductive toxicity, local respiratory toxicity, phototoxicity/photoallergenicity, skin sensitization, and environmental safety. Data show that 2-methyltetrahydrofuran-3-one is not genotoxic. The repeated dose, reproductive, and local respiratory toxicity endpoints were evaluated using the Threshold of Toxicological Concern (TTC) for a Cramer Class II material, and the exposure to 2-methyltetrahydrofuran-3-one is below the TTC (0.009 mg/kg/day, 0.009 mg/kg/day, and 0.47 mg/day, respectively). The skin sensitization endpoint was completed using the Dermal Sensitization Threshold (DST) for non-reactive materials (900 μg/cm2); exposure is below the DST.[2]
No skin sensitization studies are available for 2-methyltetrahydrofuran-3-one. The chemical structure of this material indicates that it would not be expected to react with skin proteins directly (Roberts et al., 2007; Toxtree v3.1.0; OECD Toolbox v4.2). Due to the lack of data, the reported exposure was benchmarked utilizing the non-reactive DST of 900 μg/cm2. The current exposure from the 95th percentile concentration is below the DST for non-reactive/reactive materials when evaluated in all QRA categories.
Here provides the maximum acceptable concentrations for 2-methyltetrahydrofuran-3-one that present no appreciable risk for skin sensitization based on the non-reactive DST. These levels represent maximum acceptable concentrations based on the DST approach. However, additional studies may show it could be used at higher levels.
References
[1] Eulália L. S. Barros, Diego A. M., Evertan A. Rebelatto, & J. Vladimir Oliveira*, (2024). Phase Equilibrium and Thermodynamic Modeling of 2-Methyltetrahydrofuran-3-one, Carbon Dioxide, and Lauric Acid Systems. Journal of Chemical & Engineering Data, 69 10, 3472-3480 3472-3480.
[2]Api AM, Belsito D, Botelho D, Bruze M, Burton GA Jr, Cancellieri MA, Chon H, Dagli ML, Date M, Dekant W, Deodhar C, Fryer AD, Jones L, Joshi K, Kumar M, Lapczynski A, Lavelle M, Lee I, Liebler DC, Moustakas H, Na M, Penning TM, Ritacco G, Romine J, Sadekar N, Schultz TW, Selechnik D, Siddiqi F, Sipes IG, Sullivan G, Thakkar Y, Tokura Y. RIFM fragrance ingredient safety assessment, 2-methyltetrahydrofuran-3-one, CAS Registry Number 3188-00-9. Food Chem Toxicol. 2022 Dec;169 Suppl 1:113466. doi: 10.1016/j.fct.2022.113466. Epub 2022 Oct 10. PMID: 36228899.
You may like
Lastest Price from 2-Methyltetrahydrofuran-3-one manufacturers
US $0.00-0.00/KG2025-06-26
- CAS:
- 3188-00-9
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 10000KGS

US $50.00/kg2025-04-21
- CAS:
- 3188-00-9
- Min. Order:
- 1kg
- Purity:
- 99
- Supply Ability:
- 5000