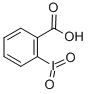
2-Iodoxybenzoic acid-a hypervalent iodine oxidant
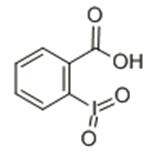
As a typically hypervalent iodine reagents, 2-iodoxybenzoic acid (IBX) was widely used as an oxidant in organic synthesis to oxidize hydroxyl to carbonyl. The compounds were first synthesized in 1893, but because of its poor solubility in common organic solvents, IBX was not widely used at first. In the year of 1994, Frigerio found it soluble in dimethyl sulfoxide, oxidation of alcohol and adjacent diol effect is very good, thus revealed IBX application of a new chapter in the organic synthesis.
There are two tautologous isomers, one of which is a carboxylic acid structure.The pKa of IBX is 2.4 in water and 6.65 in DMSO. Its acidity also leads to acid-catalyzed isomerization as an oxidation side reaction. It could be prepared from o-iodobenzoic acid, potassium bromate (or potassium bisulfite compound salt) and sulfuric acid. It is stable in air and can be stored for a long time.
Oxidation of alcohols to aldehydes employing IBX as oxidant undergoes a so-called hypervalent torsion mechanism, which involves ligand exchange (replacing alcohol hydroxyl groups), torsion, and elimination. In the torsional step the oxygen atom is moved to the transition state of the five-membered ring required for the elimination reaction. The driving force of torsion is the steric hindrance between alkoxy group proton and aromatic ring ortho proton. Therefore, higher alcohols are more easily oxidized by IBX than lower alcohols. Calculations also show that if the ortho proton is replaced with a methyl group, the reaction rate will be increased to 100 times of the former. Torsion is the speed-controlled step of the reaction and is necessary, otherwise iodine-oxygen double bond and alkoxy group are noncoplanar, and collaborative elimination cannot occur.
IBX can also be loaded onto silicone or polystyrene. Compared with IBX, these polymers containing IBX have the advantages of simple separation, reagents recycling and no explosion, etc. Moreover, their oxidation properties are similar to IBX, and satisfactory results can be obtained. The mixture of IBX and acetic acid and acetic anhydride is heated to give the Dess-Martin Periodinane (DMP), which is more soluble in common organic solvents and has a wide range of applications. IBX often has similar properties to DMP in oxidation, but DMP is unstable and cannot be stored for a long time.
References
[1] Qin, K.-Y.; Su, G.-F.; Rao, W.-P.; Tan, G.-M. Application of 2-Iodoxybenzoic Acid (IBX) in Organic Synthesis, Chinese Journal of Organic Chemistry, 2006, 26 (12): 1623-1630.
[2] Ballaschk, F.; Kirsch, S. F. Oxidation of secondary alcohols using solidsupported hypervalent iodine catalysts, Green Chem., 2019, 21, 5896-5903.
[3] Zhang, Z.; Li, X.; Song, M.; et al. Selective Removal of Aminoquinoline Auxiliary by IBX Oxidation, J. Org. Chem., 2019, 84(20): 12792-12799.
[4] Ambule, M. D.; Tripathi, S.; Ghoshal, A.; et al. IBX-mediated oxidative addition of isocyanides to cyclic secondary amines: total syntheses of alangiobussine and alangiobussinine, Chem. Commun., 2019, 55, 10872-10875.
You may like
See also
Lastest Price from 2-Iodoxybenzoic acid manufacturers

US $0.00-0.00/kg2025-04-21
- CAS:
- 61717-82-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 20MT

US $0.00/kg2024-04-12
- CAS:
- 61717-82-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000ton