2-Iodobenzoic Acid: Applications in Medicinal Chemistry and its Preparation Method
General Description
The versatility of 2-Iodobenzoic acid in medicinal chemistry is evident from its role in synthesizing radioiodinated esters and amides for adrenal imaging agents, as well as in the development of N-hydroxypyridone derivatives with potential anti-ischemic stroke properties. Its significance lies in enhancing diagnostic techniques and potential treatments. The synthesis method involves starting with reactants including 2-oxo-1,2-diphenylethyl 2-iodobenzoate, using specific catalysts and conditions under blue LED irradiation to ensure a high yield of 100% for the final product. This method enables the production of compounds crucial for medical advancements, demonstrating the pivotal role of 2-Iodobenzoic acid in driving innovation in medicinal chemistry.

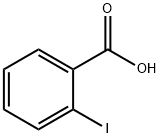
Figure 1. 2-Iodobenzoic acid
Applications in Medicinal Chemistry
2-Iodobenzoic acid, a versatile compound, finds applications in diverse fields, notably in medicinal chemistry. One significant application lies in the synthesis of radioiodinated esters and amides, as demonstrated in the Journal of Medicinal Chemistry. These derivatives are crucial for potential tumor or organ imaging agents, particularly in the context of adrenal imaging. In the pursuit of developing superior adrenal imaging agents, researchers synthesized radioiodinated benzoyl esters and amides of epimeric 20-hydroxy- and 20-aminopregn-5-en-3β-ols. The process involved treating the appropriate epimer with 2-iodobenzoic acid in the presence of specific reagents. The resulting esters and amides were then readily labeled with radioiodine, enabling their utilization in imaging studies. Tissue distribution studies in female rats revealed the significant adrenal specificity of these compounds, particularly the esters. Importantly, the ester with a similar configuration at C-20 as cholesterol exhibited superior performance compared to its epimer. This underscores the potential of 2-Iodobenzoic acid-derived compounds in enhancing adrenal imaging capabilities, thus advancing diagnostic techniques in clinical settings. Furthermore, 2-Iodobenzoic acid contributes to the development of novel N-hydroxypyridone derivatives with potential anti-ischemic stroke properties. Based on the promising lead compound ciclopirox (CPX), a series of derivatives were designed, synthesized, and evaluated. Among these, compound I emerged as a significant neuroprotective agent against ischemic stroke-induced injuries in neuronal cells. Compound I demonstrated favorable characteristics such as good blood-brain barrier permeability, superior antioxidant capability, and negligible hERG inhibition. Moreover, its complex with olamine exhibited enhanced water solubility and metabolic stability. In vivo experiments confirmed the efficacy of compound I·olamine in reducing brain infarction and alleviating neurological deficits in rats with middle cerebral artery occlusion. In summary, 2-Iodobenzoic acid serves as a pivotal building block for the synthesis of compounds with diverse applications in medicinal chemistry, ranging from adrenal imaging agents to potential treatments for ischemic stroke. Its versatility and efficacy highlight its significance in advancing research and innovation in the field of medicine. 1,2
Preparation Method
To prepare 2-Iodobenzoic acid, start with reactants including 2-oxo-1,2-diphenylethyl 2-iodobenzoate. In a reaction tube, add [Ru(bpy)3](PF6)2 catalyst (1.0 mol %), L-ascorbic acid (1.5 equivalents), and tripotassium phosphate (1 equivalent) to the reactant. Dissolve the mixture in an acetonitrile/water mixture (4:1 v/v; 0.17M). Under blue LED irradiation at room temperature and vigorous stirring for 1 hour, the reaction proceeds. Then, pour the mixture into water and extract it with ethyl acetate. Acidify the water layer with aqueous potassium bisulfate (1M) to pH 2-3. Extract the mixture again with ethyl acetate multiple times, combine the organic layers, and dry them over Na2SO4. After filtration, remove the solvent under reduced pressure. Next, purify the residue by column chromatography using silica gel, with a hexane/acetone solvent mixture (9:1 ratio) until only a baseline spot remains. Then, add triethyl orthoformiate (5%) and formic acid (1%) to the solvent mixture. Finally, for transformation, consider hydrolysis or hydrogenolysis of carboxylic esters or thioesters to achieve the desired product, 2-Iodobenzoic acid. This method ensures a high yield of 100% for the final product. 3
Reference
1. Haradahira T, Schwendner SW, Kojima M, Counsell RE. Potential tumor- or organ-imaging agents. 29. Radioiodinated esters and amides of 20-hydroxy- and 20-aminopregn-5-en-3 beta-ols. J Med Chem. 1989; 32(3): 609-612.
2. Hu L, Feng H, Zhang H, et al. Development of Novel N-hydroxypyridone Derivatives as Potential Anti-Ischemic Stroke Agents. J Med Chem. 2020; 63(3): 1051-1067.
3. Elisabeth S, Kirsten Z. Desyl and Phenacyl as Versatile, Photocatalytically Cleavable Protecting Groups: A Classic Approach in a Different (Visible) Light. ACS Catalysis. 2017; 7(10): 6821–6826.
Related articles And Qustion
Lastest Price from 2-Iodobenzoic acid manufacturers

US $0.00/KG2025-04-21
- CAS:
- 88-67-5
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $0.00-0.00/KG2025-04-21
- CAS:
- 88-67-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 mt